The advantages of endophyte-infected over uninfected tall fescue in the growth and pathogen resistance are counteracted by elevated CO2
- PMID: 28761108
- PMCID: PMC5537266
- DOI: 10.1038/s41598-017-07183-y
The advantages of endophyte-infected over uninfected tall fescue in the growth and pathogen resistance are counteracted by elevated CO2
Abstract
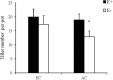
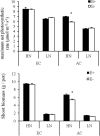
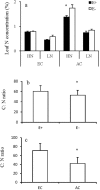
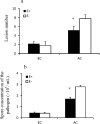
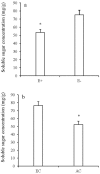
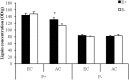
Atmospheric CO2 concentrations are predicted to double within the next century. Despite this trend, the extent and mechanisms through which elevated CO2 affects grass-endophyte symbionts remain uncertain. In the present study, the growth, chemical composition and pathogen resistance of endophyte-infected (E+) and uninfected (E-) tall fescue were compared under elevated CO2 conditions. The results showed that the effect of endophyte infection on the growth of tall fescue was significantly affected by elevated CO2. Significant advantage of E+ over E- tall fescue in tiller number, maximum net photosynthetic rate and shoot biomass occurred only under ambient CO2. With CO2 concentration elevated, the beneficial effect of endophyte infection on the growth disappeared. Similarly, endophyte infection reduced lesion number and spore concentration of Curvularia lunata only under ambient CO2. These results suggest that the beneficial effect of endophyte infection on the growth and pathogen resistance of tall fescue could be counteracted by elevated CO2. An explanation for the counteraction may be found in a change in photosynthesis and nutritive quality of leaf tissue.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (ed. by T. F. Stocker, D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley), pp. 148. Cambridge University Press, Cambridge, U. K. and New York, New York. doi:10.5061/dryad.56cn8015 10.1086/665032 (2013).
-
- Bryant JP, Chapin FS, Klein DR. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos. 1983;40:357–368. doi: 10.2307/3544308. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

