Influence of Repeated Senna Laxative Use on Skin Barrier Function in Mice
- PMID: 28761288
- PMCID: PMC5500705
- DOI: 10.5021/ad.2017.29.4.414
Influence of Repeated Senna Laxative Use on Skin Barrier Function in Mice
Abstract
Background: Senna, one of the major stimulant laxatives, is widely used for treating constipation. Chronic senna use has been reported to be associated with colonic disorders such as melanosis coli and/or epithelial hyperplasia. However, there is no obvious information on the influence of chronic senna use on organs except for the intestine.
Objective: To clarify the influence of senna laxative use on skin barrier function by repeated senna administration.
Methods: Eight-week-old male hairless mice received senna (10 mg/kg/day) for 21 days. After administration, we evaluated transepidermal water loss (TEWL), and investigated the biomarkers in plasma and skin using protein analysis methods.
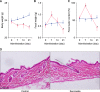
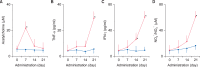
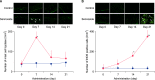
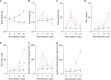
Results: Fecal water content on day seven was significantly increased; however, on day 21, it was significantly decreased after repeated senna administration. In the senna-administered group, TEWL was significantly higher compared to the control on days seven and 21. Plasma acetylcholine concentration and NO2-/NO3- were increased on days seven and 21, respectively. In skin, tryptase-positive mast cells and inducible nitric oxide synthase (iNOS)-positive cells were increased on days seven and 21, respectively. The increase of TEWL on days seven and 21 was suppressed by the administration of atropine and N(G)-nitro-L-arginine methyl ester, respectively.
Conclusion: It was suggested that diarrhea or constipation induced by repeated senna administration caused the impairment of skin barrier function. There is a possibility that this impaired skin barrier function occurred due to degranulation of mast cells via cholinergic signals or oxidative stress derived from iNOS.
Keywords: Acetylcholine; Nitric oxide synthase type II; Senna extract; Skin hydration; Transepidermal water loss.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors have nothing to disclose.
Figures
Similar articles
-
Are Senna based laxatives safe when used as long term treatment for constipation in children?J Pediatr Surg. 2018 Apr;53(4):722-727. doi: 10.1016/j.jpedsurg.2018.01.002. Epub 2018 Jan 31. J Pediatr Surg. 2018. PMID: 29429768 Review.
-
Colonoscopic Resolution of Melanosis Coli After Cessation of Senna Laxative Use.Int Med Case Rep J. 2024 Sep 11;17:783-787. doi: 10.2147/IMCRJ.S475869. eCollection 2024. Int Med Case Rep J. 2024. PMID: 39282237 Free PMC article.
-
Impairment of skin barrier function via cholinergic signal transduction in a dextran sulphate sodium-induced colitis mouse model.Exp Dermatol. 2015 Oct;24(10):779-84. doi: 10.1111/exd.12775. Epub 2015 Aug 18. Exp Dermatol. 2015. PMID: 26014805
-
Immunological changes in the intestines and skin after senna administration.Pharm Biol. 2015 Jun;53(6):913-20. doi: 10.3109/13880209.2014.948636. Epub 2014 Nov 28. Pharm Biol. 2015. PMID: 25430604
-
Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review).Evid Based Child Health. 2013 Jan;8(1):57-109. doi: 10.1002/ebch.1893. Evid Based Child Health. 2013. PMID: 23878124 Review.
References
-
- Wald A, Scarpignato C, Mueller-Lissner S, Kamm MA, Hinkel U, Helfrich I, et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther. 2008;28:917–930. - PubMed
-
- Yamate Y, Hiramoto K, Yokoyama S, Ooi K. Immunological changes in the intestines and skin after senna administration. Pharm Biol. 2015;53:913–920. - PubMed
-
- Matsui T, Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol. 2015;27:269–280. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

