Chronic constriction injury of sciatic nerve changes circular RNA expression in rat spinal dorsal horn
- PMID: 28761373
- PMCID: PMC5522680
- DOI: 10.2147/JPR.S139592
Chronic constriction injury of sciatic nerve changes circular RNA expression in rat spinal dorsal horn
Abstract
Background: Mechanisms of neuropathic pain are still largely unknown. Molecular changes in spinal dorsal horn may contribute to the initiation and development of neuropathic pain. Circular RNAs (circRNAs) have been identified as microRNA sponges and involved in various biological processes, but whether their expression profile changes in neuropathic pain condition is not reported.
Methods: To test whether neuropathic pain influences circRNA expression, we developed a sciatic chronic constriction injury (CCI) model in rats. The CCI ipsilateral spinal dorsal horns of lumbar enlargement segments (L3-L5) were collected, and the total RNA was extracted and subjected to Arraystar Rat circRNA Microarray. Quantitative real-time polymerase chain reaction (qPCR) was used to confirm the circRNA expression profile. To estimate functions of differential circRNAs, bioinformatics analyses including gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes Pathway analyses were performed for the top 100 circRNAs and circRNA-microRNA networks were constructed for the top 10 circRNAs.
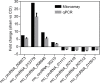
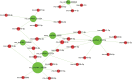
Results: circRNA microarrays showed that 469 circRNAs were differentially expressed between CCI and sham-operated rats (fold change ≥2). In all, 363 of them were significantly upregulated, and the other 106 were downregulated in the CCI group. Three of them (circRNA_013779, circRNA_008008, and circRNA_003724) overexpressed >10 times after CCI insult. Expression levels of eight circRNAs were verified using qPCR. GO analysis revealed that thousands of predicted target genes were involved in the biological processes, cellular component, and molecular function; in addition, dozens of these genes were enriched in the Hippo signaling pathway, MAPK signaling pathway, and so on. Competing endogenous RNAs analysis showed that circRNA_008008 and circRNA_013779 are the two largest nodes in the circRNA-microRNA interaction network of the top 10 circRNAs.
Conclusion: CCI resulted in a comprehensive expression profile of circRNAs in the spinal dorsal horn in rats. CircRNAs in the dorsal horn could be helpful to reveal molecular mechanisms of neuropathic pain.
Keywords: circRNA–microRNA interaction; circular RNA; microarray; neuropathic pain.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Van HO, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. - PubMed
-
- Loeser JD. Economic implications of pain management. Acta Anaesthesiol Scand. 1999;43(9):957–959. - PubMed
-
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

