The crossed phrenic phenomenon
- PMID: 28761411
- PMCID: PMC5514853
- DOI: 10.4103/1673-5374.208539
The crossed phrenic phenomenon
Abstract
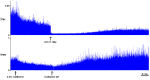
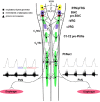
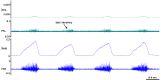
The cervical spine is the most common site of traumatic vertebral column injuries. Respiratory insufficiency constitutes a significant proportion of the morbidity burden and is the most common cause of mortality in these patients. In seeking to enhance our capacity to treat specifically the respiratory dysfunction following spinal cord injury, investigators have studied the "crossed phrenic phenomenon", wherein contraction of a hemidiaphragm paralyzed by a complete hemisection of the ipsilateral cervical spinal cord above the phrenic nucleus can be induced by respiratory stressors and recovers spontaneously over time. Strengthening of latent contralateral projections to the phrenic nucleus and sprouting of new descending axons have been proposed as mechanisms contributing to the observed recovery. We have recently demonstrated recovery of spontaneous crossed phrenic activity occurring over minutes to hours in C1-hemisected unanesthetized decerebrate rats. The specific neurochemical and molecular pathways underlying crossed phrenic activity following injury require further clarification. A thorough understanding of these is necessary in order to develop targeted therapies for respiratory neurorehabilitation following spinal trauma. Animal studies provide preliminary evidence for the utility of neuropharmacological manipulation of serotonergic and adenosinergic pathways, nerve grafts, olfactory ensheathing cells, intraspinal microstimulation and a possible role for dorsal rhizotomy in recovering phrenic activity following spinal cord injury.
Keywords: C1; C2; SCI; cervical; diaphragm; hemidiaphragm; hemisection; neuroplasticity; paralysis; phrenic; recovery; respiratory; spinal cord injury.
Conflict of interest statement
Conflicts of interest: None declared.
Figures

Similar articles
-
Dynamic changes in phrenic motor output following high cervical hemisection in the decerebrate rat.Exp Neurol. 2015 Sep;271:379-89. doi: 10.1016/j.expneurol.2015.06.002. Epub 2015 Jun 6. Exp Neurol. 2015. PMID: 26056711
-
The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury.J Appl Physiol (1985). 2003 Feb;94(2):795-810. doi: 10.1152/japplphysiol.00847.2002. J Appl Physiol (1985). 2003. PMID: 12531916 Review.
-
Identification of the neural pathway underlying spontaneous crossed phrenic activity in neonatal rats.Neuroscience. 2009 Nov 10;163(4):1109-18. doi: 10.1016/j.neuroscience.2009.07.011. Epub 2009 Jul 31. Neuroscience. 2009. PMID: 19596054 Free PMC article.
-
Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat.Exp Neurol. 1992 Jun;116(3):219-28. doi: 10.1016/0014-4886(92)90001-7. Exp Neurol. 1992. PMID: 1375167
-
The bulbospinal network controlling the phrenic motor system: Laterality and course of descending projections.Neurosci Res. 2017 Aug;121:7-17. doi: 10.1016/j.neures.2017.03.004. Epub 2017 Apr 4. Neurosci Res. 2017. PMID: 28389264 Review.
Cited by
-
Pharmacological modulation of respiratory control: Ampakines as a therapeutic strategy.Pharmacol Ther. 2025 Jan;265:108744. doi: 10.1016/j.pharmthera.2024.108744. Epub 2024 Nov 8. Pharmacol Ther. 2025. PMID: 39521442 Review.
-
Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats.Biology (Basel). 2022 Mar 19;11(3):473. doi: 10.3390/biology11030473. Biology (Basel). 2022. PMID: 35336846 Free PMC article.
-
Electrical epidural stimulation of the cervical spinal cord: implications for spinal respiratory neuroplasticity after spinal cord injury.J Neurophysiol. 2021 Aug 1;126(2):607-626. doi: 10.1152/jn.00625.2020. Epub 2021 Jul 7. J Neurophysiol. 2021. PMID: 34232771 Free PMC article. Review.
-
Effects of C2 hemisection on respiratory and cardiovascular functions in rats.Neural Regen Res. 2023 Feb;18(2):428-433. doi: 10.4103/1673-5374.346469. Neural Regen Res. 2023. PMID: 35900441 Free PMC article.
-
Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation.Cells. 2023 May 31;12(11):1519. doi: 10.3390/cells12111519. Cells. 2023. PMID: 37296640 Free PMC article. Review.
References
-
- Allan DW, Greer JJ. Development of phrenic motoneuron morphology in the fetal rat. J Comp Neurol. 1997;382:469–479. - PubMed
-
- Arborelius M, Lilja B, Senyk J. Regional and total lung function in patients with hemidiaphragmatic paralysis. Respiration. 1975;32:253–264. - PubMed
-
- Awad BI, Warren PM, Steinmetz MP, Alilain WJ. The role of the crossed phrenic pathway after cervical contusion injury and a new model to evaluate therapeutic interventions. Exp Neurol. 2013;248:398–405. - PubMed
-
- Bareyre FM. Neuronal repair and replacement in spinal cord injury. J Neurol Sci. 2007;265:63–72. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

