Toxicity and Apoptosis Related Effects of Benzimidazo [3,2-α] Quinolinium Salts Upon Human Lymphoma Cells
- PMID: 28761559
- PMCID: PMC5510565
- DOI: 10.2174/1874104501711010054
Toxicity and Apoptosis Related Effects of Benzimidazo [3,2-α] Quinolinium Salts Upon Human Lymphoma Cells
Abstract
Objectives: The present study evaluates novel cationic quinoline derivatives known as benzimidazo[3,2-a]quinolinium salts (BQS) named NBQ-48 and ABQ-48 that have structural similarities to known anti-cancer substances such as ellipticine and berberine.
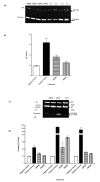
Methods: Toledo human lymphoma (ATCC CRL2631) cells were treated for 24 to 48 hours. Apoptosis related endpoints such as cell cycle arrest, mitochondrial damage, RNS and ROS generation and the activity of several apoptosis related proteins such as caspases and apoptosis inducing factor (AIF) were studied using fluorescence staining and western blot respectively.
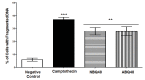
Results: Results indicated a higher toxicity from the amino substituted ABQ-48 versus the NBQ-48 (GI50's of 50uM versus 100uM respectively). Both compounds induced cell death through various apoptosis related endpoints including a decrease in mitochondrial membrane potential with an increase in ROS and activation of the effector caspase 3. Interestingly, AIF release was observed on cells treated with the amino substituted ABQ-48 but not on the nitro substituted NBQ-48 samples suggesting a caspase independent mechanism for ABQ-48.
Conclusions: The results obtained presents the toxic effects of two novel benzimidazo[3,2-a]quinolinium salts in human lymphoma tumor cells. The identified mechanism of action includes multiple apoptosis related effects. Furthermore the data presents a clear variation in caspase dependent or independent mechanism for each compound.
Keywords: Anti cancer; Apoptosis Inducing Factor; Apotosis; BQS; Benzimidazo[3,2-a]quinolinium Salts; Diffuse large B-cell Lymphoma; Unnatural alkaloid.
Figures







References
-
- Hurren R., Beheshti Zavareh R., Dalili S., Wood T., Rose D., Chang H., et al. A novel diquinolonium displays preclinical anti-cancer activity and induces caspase-independent cell death. Apoptosis : An Inter. J. on Programmed Cell Death. 2008;13(6):748–755. doi: 10.1007/s10495-008-0209-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
