Modified Atkins diet in adult with refractory epilepsy: A controlled randomized clinical trial
- PMID: 28761628
- PMCID: PMC5526780
Modified Atkins diet in adult with refractory epilepsy: A controlled randomized clinical trial
Abstract
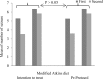
Background: The usefulness of the modified Atkins diet (mAD) in refractory epilepsy in adults has been rarely investigated. We aimed to evaluate the efficacy of mAD in adult with refractory epilepsy. Methods: In a controlled randomized clinical trial, we enrolled 66 refractory adult epileptic cases from February 2010 to December 2012. The patients were randomly divided into two groups, case groups (22 patients) used antiepileptic drugs and mAD and control group (32 patients) only use antiepileptic drugs. The primary outcome was at least 50% decrement in seizure frequency after 2 months of therapy. Results: No significant difference was shown in our data between groups regarding baseline characteristic. The differences of mean seizure attack after 2 months (P < 0.001). (17.6%) had > 50% seizure decrease at 1 and after 2 months and 12 (35.3%) had 50% decrease in seizure frequency. Furthermore, in mAD group, the mean urinary ketone positivity was 1.75 ± 0.28 and increasing liver enzyme was shown 5 cases (14.7%) in mAD group and 5 cases (15.6%) in control group (P < 0.050). Conclusion: The mAD may be effective as a cotherapy treatment for adults with refractory epilepsy and decrease 2.19 times seizure frequency in comparison with control groups. Trials with the more tolerant dietary regime, with larger sample size and longer duration, should be performed in future.
Keywords: Adult; Drug Refractory; Epilepsy; Modified Atkins Diet.
Figures
References
-
- Kossoff EH. More fat and fewer seizures: dietary therapies for epilepsy. Lancet Neurol. 2004;3(7):415–20. - PubMed
-
- Kossoff EH, Dorward JL. The modified Atkins diet. Epilepsia. 2008;49(Suppl 8):37–41. - PubMed
-
- Kossoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61(12):1789–91. - PubMed
-
- Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421–4. - PubMed
-
- Kossoff EH, Turner Z, Bluml RM, Pyzik PL, Vining EP. A randomized, crossover comparison of daily carbohydrate limits using the modified Atkins diet. Epilepsy Behav. 2007;10(3):432–6. - PubMed
LinkOut - more resources
Full Text Sources