Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma
- PMID: 28761735
- PMCID: PMC5519810
- DOI: 10.1136/esmoopen-2017-000168
Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma
Abstract
Objective: Oral tyrosine kinase inhibitor has been shown to prolong progression-free survival (PFS) in estimated glomerular filtration rate (EGFR) mutation positive adenocarcinoma; however, the comparator arm has not included the current standard adenocarcinoma regimen (pemetrexed carboplatin induction followed by maintenance pemetrexed) and patients from Indian subcontinent. Hence, this study was carried out in Indian patients to compare gefitinib with the above-mentioned chemotherapy regimen.
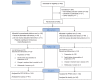
Methods: This was an open-labelled, randomised, parallel group study comparing gefitinib (250 mg orally daily) with pemetrexed (500 mg/m2) and carboplatin (area under the curve 5) doublet intravenous induction chemotherapy regimen followed by maintenance pemetrexed (500 mg/m2) in patients with EGFR-activating mutation-positive stage IIIB or stage IV adenocarcinoma lung in the first-line setting. The primary endpoint for the study was PFS. 260 patients were required to demonstrate a 50% improvement in PFS of gefitinib over chemotherapy, with 80% power and 5% type 1 error. With an expected 5% dropout rate, the sample size was 290 patients.
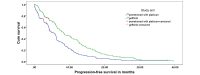
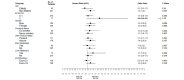
Results: The median PFS in gefitinib arm was 8.4 months (95% CI 6.3 to 10.5 months) compared with 5.6 months (95% CI 4.2 to 7.0 months) in pemetrexed-carboplatin arm (HR: 95% CI 0.513 to 0.851; p -0.001). The impact of gefitinib on PFS was seen across all subgroups.There was no statistically significant difference in overall survival between the two arms. Haematologicalgrade3-4toxicities likeanaemia,neutropaenia and thrombocytopaenia were common in the pemetrexed-carboplatin arm while grade3-4 acneiform rash and diarrhoeawere common in the gefitinib arm.
Conclusion: The study confirms the superiority of gefitinib in prolonging PFS against the most active chemotherapy regimen of pemetrexed-carboplatin followed by maintenance pemetrexed in EGFR-mutated lung adenocarcinoma. The median PFS in Indian patients in gefitinib arm is similar to that reported in east Asians and Caucasians.
Keywords: EGFR mutation; Gefitinib; Lung cancer; NSCLC; Palliative; Pemetrexed.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Rosell R, Carcereny E, Gervais R, et al. ; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. 10.1016/S1470-2045(11)70393-X - DOI - PubMed
-
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. 10.1016/S1470-2045(11)70184-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

