Real-time monitoring of superoxide anion radical generation in response to wounding: electrochemical study
- PMID: 28761775
- PMCID: PMC5527980
- DOI: 10.7717/peerj.3050
Real-time monitoring of superoxide anion radical generation in response to wounding: electrochemical study
Abstract
Background: The growth and development of plants is deleteriously affected by various biotic and abiotic stress factors. Wounding in plants is caused by exposure to environmental stress, mechanical stress, and via herbivory. Typically, oxidative burst in response to wounding is associated with the formation of reactive oxygen species, such as the superoxide anion radical (O2•-), hydrogen peroxide (H2O2) and singlet oxygen; however, few experimental studies have provided direct evidence of their detection in plants. Detection of O2•- formation in plant tissues have been performed using various techniques including electron paramagnetic resonance spin-trap spectroscopy, epinephrine-adrenochrome acceptor methods, staining with dyes such as tetrazolium dye and nitro blue tetrazolium (NBT); however, kinetic measurements have not been performed. In the current study, we provide evidence of O2•- generation and its kinetics in the leaves of spinach (Spinacia oleracea) subjected to wounding.
Methods: Real-time monitoring of O2•- generation was performed using catalytic amperometry. Changes in oxidation current for O2•- was monitored using polymeric iron-porphyrin-based modified carbon electrodes (φ = 1 mm) as working electrode with Ag/AgCl as the reference electrode.
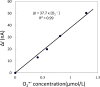
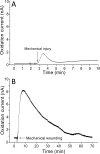
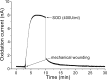
Result: The results obtained show continuous generation of O2•- for minutes after wounding, followed by a decline. The exogenous addition of superoxide dismutase, which is known to dismutate O2•- to H2O2, significantly suppressed the oxidation current.
Conclusion: Catalytic amperometric measurements were performed using polymeric iron-porphyrin based modified carbon electrode. We claim it to be a useful tool and a direct method for real-time monitoring and precise detection of O2•- in biological samples, with the potential for wide application in plant research for specific and sensitive detection of O2•-.
Keywords: Electrochemical detection; Polymeric iron-porphyrin-based modified carbon electrode; Superoxide anion radical; Wounding.
Conflict of interest statement
Ryo Matsuoka and Shigeo Aoyagi are employees of Hokuto Denko Corporation, Tokyo, Japan, and bear no competing interests. In addition, all other authors declare there are no competing interests.
Figures



Similar articles
-
Reactive Oxygen Species as a Response to Wounding: In Vivo Imaging in Arabidopsis thaliana.Front Plant Sci. 2020 Jan 9;10:1660. doi: 10.3389/fpls.2019.01660. eCollection 2019. Front Plant Sci. 2020. PMID: 31998345 Free PMC article.
-
Mechanism of horseradish peroxidase catalyzed epinephrine oxidation: obligatory role of endogenous O2- and H2O2.Biochemistry. 1998 Dec 1;37(48):16922-33. doi: 10.1021/bi980899l. Biochemistry. 1998. PMID: 9836585
-
Detection of hydrogen peroxide in Photosystem II (PSII) using catalytic amperometric biosensor.Front Plant Sci. 2015 Oct 15;6:862. doi: 10.3389/fpls.2015.00862. eCollection 2015. Front Plant Sci. 2015. PMID: 26528319 Free PMC article.
-
[Free oxygen radiacals and kidney diseases--part I].Med Pregl. 2000 Sep-Oct;53(9-10):463-74. Med Pregl. 2000. PMID: 11320727 Review. Croatian.
-
Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence.Cell Biochem Biophys. 2001;34(2):237-56. doi: 10.1385/CBB:34:2:237. Cell Biochem Biophys. 2001. PMID: 11898866 Review.
Cited by
-
Reactive Oxygen Species as a Response to Wounding: In Vivo Imaging in Arabidopsis thaliana.Front Plant Sci. 2020 Jan 9;10:1660. doi: 10.3389/fpls.2019.01660. eCollection 2019. Front Plant Sci. 2020. PMID: 31998345 Free PMC article.
References
-
- Alessandro A, Osto LD, Aprile A, Carillo P, Roncaglia E, Cattivelli L, Bassi R. Reactive oxygen species and transcript analysis upon excess light treatment in wild-type Arabidopsis thaliana vs a photosensitive mutant lacking zeaxanthin and lutein. BMC Plant Biology. 2011;11:62. doi: 10.1186/1471-2229-11-62. - DOI - PMC - PubMed
-
- Anderson AA, Rogers K, Tepper CS, Blee K, Cardon J. Timing of molecular events following elicitor treatment of plant cells. Physiology and Molecular Plant Pathology. 1991;38:1–13. doi: 10.1016/S0885-5765(05)80139-0. - DOI
-
- Asada K. Radical production and scavenging in the chloroplasts. In: Baker NR, editor. Photosynthesis and the environment. Kluwer; Dordrecht: 1996. pp. 123–150.
LinkOut - more resources
Full Text Sources
Other Literature Sources

