Rapid-Acting and Human Insulins: Hexamer Dissociation Kinetics upon Dilution of the Pharmaceutical Formulation
- PMID: 28762200
- PMCID: PMC5643355
- DOI: 10.1007/s11095-017-2233-0
Rapid-Acting and Human Insulins: Hexamer Dissociation Kinetics upon Dilution of the Pharmaceutical Formulation
Abstract
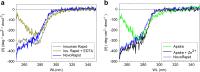
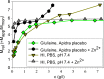
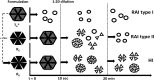
Purpose: Comparison of the dissociation kinetics of rapid-acting insulins lispro, aspart, glulisine and human insulin under physiologically relevant conditions.
Methods: Dissociation kinetics after dilution were monitored directly in terms of the average molecular mass using combined static and dynamic light scattering. Changes in tertiary structure were detected by near-UV circular dichroism.
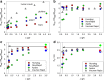
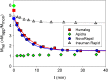
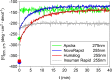
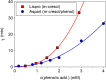
Results: Glulisine forms compact hexamers in formulation even in the absence of Zn2+. Upon severe dilution, these rapidly dissociate into monomers in less than 10 s. In contrast, in formulations of lispro and aspart, the presence of Zn2+ and phenolic compounds is essential for formation of compact R6 hexamers. These slowly dissociate in times ranging from seconds to one hour depending on the concentration of phenolic additives. The disadvantage of the long dissociation times of lispro and aspart can be diminished by a rapid depletion of the concentration of phenolic additives independent of the insulin dilution. This is especially important in conditions similar to those after subcutaneous injection, where only minor dilution of the insulins occurs.
Conclusion: Knowledge of the diverging dissociation mechanisms of lispro and aspart compared to glulisine will be helpful for optimizing formulation conditions of rapid-acting insulins.
Keywords: circular dichroism; dissociation kinetics; insulin analog; light scattering; rapid-acting.
Figures






Similar articles
-
Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature.J Pharm Sci. 2014 Aug;103(8):2255-67. doi: 10.1002/jps.24039. Epub 2014 Jun 6. J Pharm Sci. 2014. PMID: 24909933
-
Investigation of the Physico-Chemical Properties that Enable Co-Formulation of Basal Insulin Degludec with Fast-Acting Insulin Aspart.Pharm Res. 2015 Jul;32(7):2250-8. doi: 10.1007/s11095-014-1614-x. Epub 2015 Jan 8. Pharm Res. 2015. PMID: 25563978 Free PMC article.
-
Intrinsic fibrillation of fast-acting insulin analogs.J Diabetes Sci Technol. 2012 Mar 1;6(2):265-76. doi: 10.1177/193229681200600209. J Diabetes Sci Technol. 2012. PMID: 22538135 Free PMC article.
-
Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid-acting insulin analogues-aspart, lispro, and glulisine.Endocr Pract. 2011 Mar-Apr;17(2):271-80. doi: 10.4158/EP10260.RA. Endocr Pract. 2011. PMID: 21134878 Review.
-
Clinical pharmacokinetics and pharmacodynamics of insulin lispro mixtures.Clin Pharmacokinet. 2002;41(13):1043-57. doi: 10.2165/00003088-200241130-00003. Clin Pharmacokinet. 2002. PMID: 12403642 Review.
Cited by
-
Analysis of insulin glulisine at the molecular level by X-ray crystallography and biophysical techniques.Sci Rep. 2021 Jan 18;11(1):1737. doi: 10.1038/s41598-021-81251-2. Sci Rep. 2021. PMID: 33462295 Free PMC article.
-
An ultrafast insulin formulation enabled by high-throughput screening of engineered polymeric excipients.Sci Transl Med. 2020 Jul 1;12(550):eaba6676. doi: 10.1126/scitranslmed.aba6676. Sci Transl Med. 2020. PMID: 32611683 Free PMC article.
-
A co-formulation of supramolecularly stabilized insulin and pramlintide enhances mealtime glucagon suppression in diabetic pigs.Nat Biomed Eng. 2020 May;4(5):507-517. doi: 10.1038/s41551-020-0555-4. Epub 2020 May 11. Nat Biomed Eng. 2020. PMID: 32393892 Free PMC article.
-
NMR Based Methods for Metabolites Analysis.Anal Chem. 2025 Mar 18;97(10):5393-5406. doi: 10.1021/acs.analchem.4c06477. Epub 2025 Mar 6. Anal Chem. 2025. PMID: 40048643 Free PMC article. Review.
-
Pharmacokinetic Analysis of Epithelial/Endothelial Cell Barriers in Microfluidic Bilayer Devices with an Air-Liquid Interface.Micromachines (Basel). 2020 May 25;11(5):536. doi: 10.3390/mi11050536. Micromachines (Basel). 2020. PMID: 32466113 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

