NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates normoxic HIF-1α and p27Kip1 expression in malignant melanoma and other human tumors
- PMID: 28762556
- PMCID: PMC5675809
- DOI: 10.1002/mc.22708
NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates normoxic HIF-1α and p27Kip1 expression in malignant melanoma and other human tumors
Abstract
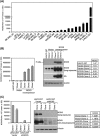
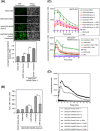
NADPH oxidase 5 (NOX5) generated reactive oxygen species (ROS) have been implicated in signaling cascades that regulate cancer cell proliferation. To evaluate and validate NOX5 expression in human tumors, we screened a broad range of tissue microarrays (TMAs), and report substantial overexpression of NOX5 in malignant melanoma and cancers of the prostate, breast, and ovary. In human UACC-257 melanoma cells that possesses high levels of functional endogenous NOX5, overexpression of NOX5 resulted in enhanced cell growth, increased numbers of BrdU positive cells, and increased γ-H2AX levels. Additionally, NOX5-overexpressing (stable and inducible) UACC-257 cells demonstrated increased normoxic HIF-1α expression and decreased p27Kip1 expression. Similarly, increased normoxic HIF-1α expression and decreased p27Kip1 expression were observed in stable NOX5-overexpressing clones of KARPAS 299 human lymphoma cells and in the human prostate cancer cell line, PC-3. Conversely, knockdown of endogenous NOX5 in UACC-257 cells resulted in decreased cell growth, decreased HIF-1α expression, and increased p27Kip1 expression. Likewise, in an additional human melanoma cell line, WM852, and in PC-3 cells, transient knockdown of endogenous NOX5 resulted in increased p27Kip1 and decreased HIF-1α expression. Knockdown of endogenous NOX5 in UACC-257 cells resulted in decreased Akt and GSK3β phosphorylation, signaling pathways known to modulate p27Kip1 levels. In summary, our findings suggest that NOX5 expression in human UACC-257 melanoma cells could contribute to cell proliferation due, in part, to the generation of high local concentrations of extracellular ROS that modulate multiple pathways that regulate HIF-1α and networks that signal through Akt/GSK3β/p27Kip1 .
Keywords: Akt/GSK3 signaling; DNA damage; NADPH oxidase; cell growth; reactive oxygen species (ROS); tumor microarray (TMA).
© 2017 Wiley Periodicals, Inc.
Figures




Similar articles
-
NOX5: Molecular biology and pathophysiology.Exp Physiol. 2019 May;104(5):605-616. doi: 10.1113/EP086204. Epub 2019 Mar 18. Exp Physiol. 2019. PMID: 30801870 Free PMC article. Review.
-
ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells.Mol Carcinog. 2016 Jan;55(1):27-39. doi: 10.1002/mc.22255. Epub 2015 Jan 5. Mol Carcinog. 2016. PMID: 25559363 Free PMC article.
-
Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody.Free Radic Biol Med. 2013 Dec;65:497-508. doi: 10.1016/j.freeradbiomed.2013.07.005. Epub 2013 Jul 11. Free Radic Biol Med. 2013. PMID: 23851018 Free PMC article.
-
NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer.Biochim Biophys Acta. 2013 Dec;1833(12):3375-3385. doi: 10.1016/j.bbamcr.2013.09.018. Epub 2013 Oct 8. Biochim Biophys Acta. 2013. PMID: 24113386
-
Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis.Antioxid Redox Signal. 2005 Sep-Oct;7(9-10):1337-45. doi: 10.1089/ars.2005.7.1337. Antioxid Redox Signal. 2005. PMID: 16115039 Review.
Cited by
-
Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis.Oxid Med Cell Longev. 2019 Sep 16;2019:8127362. doi: 10.1155/2019/8127362. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31636809 Free PMC article. Review.
-
An Integrative Gene Expression and Mathematical Flux Balance Analysis Identifies Targetable Redox Vulnerabilities in Melanoma Cells.Cancer Res. 2020 Oct 15;80(20):4565-4577. doi: 10.1158/0008-5472.CAN-19-3588. Cancer Res. 2020. PMID: 33060170 Free PMC article.
-
Endothelial NOX5 overexpression induces changes in the cardiac gene profile: potential impact in myocardial infarction?J Physiol Biochem. 2023 Nov;79(4):787-797. doi: 10.1007/s13105-023-00975-z. Epub 2023 Aug 11. J Physiol Biochem. 2023. PMID: 37566320 Free PMC article.
-
RNA methylation and cellular response to oxidative stress-promoting anticancer agents.Cell Cycle. 2023 Apr;22(8):870-905. doi: 10.1080/15384101.2023.2165632. Epub 2023 Jan 17. Cell Cycle. 2023. PMID: 36648057 Free PMC article. Review.
-
NOX5: Molecular biology and pathophysiology.Exp Physiol. 2019 May;104(5):605-616. doi: 10.1113/EP086204. Epub 2019 Mar 18. Exp Physiol. 2019. PMID: 30801870 Free PMC article. Review.
References
-
- Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double‐edged sword revisited. Annu Rev Pathol. 2014; 9:119–145. - PubMed
-
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995; 18:775–794. - PubMed
-
- Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox‐dependent signalling. Nat Rev Mol Cell Biol. 2014; 15:411–421. - PubMed
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47–95. - PubMed
-
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006; 312:1882–1883. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

