Structural dynamics of RbmA governs plasticity of Vibrio cholerae biofilms
- PMID: 28762945
- PMCID: PMC5605196
- DOI: 10.7554/eLife.26163
Structural dynamics of RbmA governs plasticity of Vibrio cholerae biofilms
Abstract
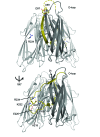
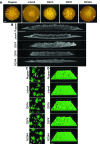
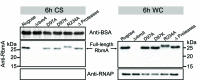
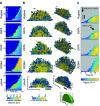
Biofilm formation is critical for the infection cycle of Vibrio cholerae. Vibrio exopolysaccharides (VPS) and the matrix proteins RbmA, Bap1 and RbmC are required for the development of biofilm architecture. We demonstrate that RbmA binds VPS directly and uses a binary structural switch within its first fibronectin type III (FnIII-1) domain to control RbmA structural dynamics and the formation of VPS-dependent higher-order structures. The structural switch in FnIII-1 regulates interactions in trans with the FnIII-2 domain, leading to open (monomeric) or closed (dimeric) interfaces. The ability of RbmA to switch between open and closed states is important for V. cholerae biofilm formation, as RbmA variants with switches that are locked in either of the two states lead to biofilms with altered architecture and structural integrity.
Keywords: RbmA; VPS; Vibrio cholerae; biofilm; biophysics; infectious disease; matrix protein; microbiology; protease; structural biology.
Conflict of interest statement
No competing interests declared.
Figures








Comment in
-
Flipping the switch.Elife. 2017 Sep 19;6:e31082. doi: 10.7554/eLife.31082. Elife. 2017. PMID: 28926339 Free PMC article.
-
Biofilms: The architect of the biofilm.Nat Rev Microbiol. 2017 Oct 12;15(11):642-643. doi: 10.1038/nrmicro.2017.127. Nat Rev Microbiol. 2017. PMID: 29021602 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

