Short-Term Effects of a Ready-to-Drink Pre-Workout Beverage on Exercise Performance and Recovery
- PMID: 28763003
- PMCID: PMC5579616
- DOI: 10.3390/nu9080823
Short-Term Effects of a Ready-to-Drink Pre-Workout Beverage on Exercise Performance and Recovery
Abstract
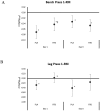
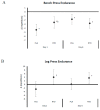
In a double-blind, randomized and crossover manner, 25 resistance-trained participants ingested a placebo (PLA) beverage containing 12 g of dextrose and a beverage (RTD) containing caffeine (200 mg), β-alanine (2.1 g), arginine nitrate (1.3 g), niacin (65 mg), folic acid (325 mcg), and Vitamin B12 (45 mcg) for 7-days, separated by a 7-10-day. On day 1 and 6, participants donated a fasting blood sample and completed a side-effects questionnaire (SEQ), hemodynamic challenge test, 1-RM and muscular endurance tests (3 × 10 repetitions at 70% of 1-RM with the last set to failure on the bench press (BP) and leg press (LP)) followed by ingesting the assigned beverage. After 15 min, participants repeated the hemodynamic test, 1-RM tests, and performed a repetition to fatigue (RtF) test at 70% of 1-RM, followed by completing the SEQ. On day 2 and 7, participants donated a fasting blood sample, completed the SEQ, ingested the assigned beverage, rested 30 min, and performed a 4 km cycling time-trial (TT). Data were analyzed by univariate, multivariate, and repeated measures general linear models (GLM), adjusted for gender and relative caffeine intake. Data are presented as mean change (95% CI). An overall multivariate time × treatment interaction was observed on strength performance variables (p = 0.01). Acute RTD ingestion better maintained LP 1-RM (PLA: -0.285 (-0.49, -0.08); RTD: 0.23 (-0.50, 0.18) kg/kgFFM, p = 0.30); increased LP RtF (PLA: -2.60 (-6.8, 1.6); RTD: 4.00 (-0.2, 8.2) repetitions, p = 0.031); increased BP lifting volume (PLA: 0.001 (-0.13, 0.16); RTD: 0.03 (0.02, 0.04) kg/kgFFM, p = 0.007); and, increased total lifting volume (PLA: -13.12 (-36.9, 10.5); RTD: 21.06 (-2.7, 44.8) kg/kgFFM, p = 0.046). Short-term RTD ingestion maintained baseline LP 1-RM (PLA: -0.412 (-0.08, -0.07); RTD: 0.16 (-0.50, 0.18) kg/kgFFM, p = 0.30); LP RtF (PLA: 0.12 (-3.0, 3.2); RTD: 3.6 (0.5, 6.7) repetitions, p = 0.116); and, LP lifting volume (PLA: 3.64 (-8.8, 16.1); RTD: 16.25 (3.8, 28.7) kg/kgFFM, p = 0.157) to a greater degree than PLA. No significant differences were observed between treatments in cycling TT performance, hemodynamic assessment, fasting blood panels, or self-reported side effects.
Keywords: dietary supplement; ergogenic aid; resistance training; sport nutrition.
Conflict of interest statement
C.P.E. serves as a paid consultant for Nutrabolt and is a Research Associate in the ESNL. Further, he holds scientific consultancies with Naturally Slim (Dallas, TX, USA) and Catapult Health (Dallas, TX, USA). R.B.K. serves as a university approved scientific advisor for Nutrabolt. P.S.M. served as quality assurance supervisor in accordance to a conflict of interest management plan that was approved by the university’s research and compliance office, the internal review board, and office of grants and contracts and monitored by research compliance. Remaining investigators have no competing interests to declare. The results from this study do not constitute endorsement by the authors and/or the institution concerning the nutrients investigated.
Figures



References
-
- Applegate E.A., Grivetti L.E. Search for the competitive edge: A history of dietary fads and supplements. J. Nutr. 1997;127:869S–873S. - PubMed
-
- Kreider R.B., Kalman D.S., Antonio J., Ziegenfuss T.N., Wildman R., Collins R., Candow D.G., Kleiner S.M., Almada A.L., Lopez H.L. International society of sports nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017;14:18. doi: 10.1186/s12970-017-0173-z. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

