Metabolic engineering of CHO cells for the development of a robust protein production platform
- PMID: 28763459
- PMCID: PMC5538670
- DOI: 10.1371/journal.pone.0181455
Metabolic engineering of CHO cells for the development of a robust protein production platform
Abstract
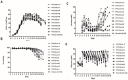
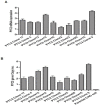
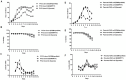
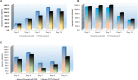
Chinese hamster ovary (CHO) cells are the most preferred mammalian host used for the bio-pharmaceutical production. A major challenge in metabolic engineering is to balance the flux of the tuned heterogonous metabolic pathway and achieve efficient metabolic response in a mammalian cellular system. Pyruvate carboxylase is an important network element for the cytoplasmic and mitochondrial metabolic pathway and efficiently contributes in enhancing the energy metabolism. The lactate accumulation in cell culture can be reduced by re-wiring of the pyruvate flux in engineered cells. In the present work, we over-expressed the yeast cytosolic pyruvate carboxylase (PYC2) enzyme in CHO cells to augment pyruvate flux towards the TCA cycle. The dual selection strategy is adopted for the screening and isolation of CHO clones containing varying number of PYC2 gene load and studied their cellular kinetics. The enhanced PYC2 expression has led to enhanced pyruvate flux which, thus, allowed reduced lactate accumulation up to 4 folds and significant increase in the cell density and culture longevity. With this result, engineered cells have shown a significant enhanced antibody expression up to 70% with improved product quality (~3 fold) as compared to the parental cells. The PYC2 engineering allowed overall improved cell performance with various advantages over parent cells in terms of pyruvate, glucose, lactate and cellular energy metabolism. This study provides a potential expression platform for a bio-therapeutic protein production in a controlled culture environment.
Conflict of interest statement
Figures



References
-
- Kim JY, Kim Y-G, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol. 2012;93: 917–930. doi: 10.1007/s00253-011-3758-5 - DOI - PubMed
-
- Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv. 2012;30: 1158–1170. doi: 10.1016/j.biotechadv.2011.08.022 - DOI - PubMed
-
- Gupta SK, Shukla P. Advanced technologies for improved expression of recombinant proteins in bacteria: perspectives and applications. Crit Rev Biotechnol. 2016;36: 1089–1098. doi: 10.3109/07388551.2015.1084264 - DOI - PubMed
-
- Gupta SK, Shukla P. Microbial platform technology for recombinant antibody fragment production: A review. Crit Rev Microbiol. 2017;43: 31–42. doi: 10.3109/1040841X.2016.1150959 - DOI - PubMed
-
- Calo-Fernández B, Martínez-Hurtado JL. Biosimilars: company strategies to capture value from the biologics market. Pharmaceuticals (Basel). 2012;5: 1393–408. doi: 10.3390/ph5121393 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

