Inhibitory effects and mechanism of dihydroberberine on hERG channels expressed in HEK293 cells
- PMID: 28763460
- PMCID: PMC5538702
- DOI: 10.1371/journal.pone.0181823
Inhibitory effects and mechanism of dihydroberberine on hERG channels expressed in HEK293 cells
Abstract
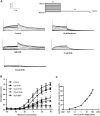
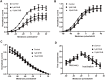
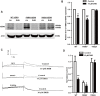
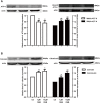
The human ether-a-go-go-related gene (hERG) potassium channel conducts rapid delayed rectifier potassium currents (IKr) and contributes to phase III cardiac action potential repolarization. Drugs inhibit hERG channels by binding to aromatic residues in hERG helixes. Berberine (BBR) has multiple actions, and its hydrogenated derivative dihydroberberine (DHB) is a potential candidate for developing new drugs. Previous studies have demonstrated that BBR blocks hERG channels and prolongs action potential duration (APD). Our present study aimed to investigate the effects and mechanism of DHB on hERG channels. Protein expression and the hERG current were analyzed using western blotting and patch-clamp, respectively. DHB inhibited the hERG current concentration-dependently after instantaneous perfusion, accelerated channel inactivation by directly binding tyrosine (Tyr652) and phenylalanine (Phe656), and decreased mature (155-kDa) and simultaneously increased immature (135-kDa) hERG expression, respectively. This suggests disruption of forward trafficking of hERG channels. Besides, DHB remarkably reduced heat shock protein 90 (Hsp90) expression and its interaction with hERG, indicating that DHB disrupted hERG trafficking by impairing channel folding. Meanwhie, DHB enhanced the expression of cleaved activating transcription factor-6 (ATF-6), a biomarker of unfolded protein response (UPR). Expression of calnexin and calreticulin, chaperones activated by ATF-6 to facilitate channel folding, were also increased, which indicating UPR activation. Additionally, the degradation rate of mature 155-kDa hERG increased following DHB exposure. In conclusion, we demonstrated that DHB acutely blocked hERG channels by binding the aromatic Tyr652 and Phe656. DHB may decrease hERG plasma membrane expression through two pathways involving disruption of forward trafficking of immature hERG channels and enhanced degradation of mature hERG channels. Furthermore, forward trafficking was disrupted by impaired channel folding associated with altered interactions between hERG proteins and chaperones. Finally, trafficking inhibition activated UPR, and mature hERG channel degradation was increased by DHB.
Conflict of interest statement
Figures





Similar articles
-
Berberine induces hERG channel deficiency through trafficking inhibition.Cell Physiol Biochem. 2014;34(3):691-702. doi: 10.1159/000363034. Epub 2014 Aug 18. Cell Physiol Biochem. 2014. PMID: 25171176
-
Intracellular Mechanism of Rosuvastatin-Induced Decrease in Mature hERG Protein Expression on Membrane.Mol Pharm. 2019 Apr 1;16(4):1477-1488. doi: 10.1021/acs.molpharmaceut.8b01102. Epub 2019 Mar 20. Mol Pharm. 2019. PMID: 30807184
-
High Glucose Represses hERG K+ Channel Expression through Trafficking Inhibition.Cell Physiol Biochem. 2015;37(1):284-96. doi: 10.1159/000430353. Epub 2015 Aug 24. Cell Physiol Biochem. 2015. PMID: 26303164
-
HERG channel trafficking.Novartis Found Symp. 2005;266:57-69; discussion 70-4, 95-9. Novartis Found Symp. 2005. PMID: 16050262 Review.
-
hERG trafficking inhibition in drug-induced lethal cardiac arrhythmia.Eur J Pharmacol. 2014 Oct 15;741:336-9. doi: 10.1016/j.ejphar.2014.06.044. Epub 2014 Jul 3. Eur J Pharmacol. 2014. PMID: 24998878 Review.
Cited by
-
Chemical composition, antioxidant, antimicrobial, and anticancer activities of Mahonia napaulensis DC. bark from Nepal.BMC Complement Med Ther. 2025 Mar 14;25(1):105. doi: 10.1186/s12906-025-04806-0. BMC Complement Med Ther. 2025. PMID: 40087695 Free PMC article.
-
Toward a broader view of mechanisms of drug cardiotoxicity.Cell Rep Med. 2021 Mar 16;2(3):100216. doi: 10.1016/j.xcrm.2021.100216. eCollection 2021 Mar 16. Cell Rep Med. 2021. PMID: 33763655 Free PMC article. Review.
-
Synthesis of new dihydroberberine and tetrahydroberberine analogues and evaluation of their antiproliferative activity on NCI-H1975 cells.Beilstein J Org Chem. 2020 Jul 6;16:1606-1616. doi: 10.3762/bjoc.16.133. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32704327 Free PMC article.
-
Visualizing semipermeability of the cell membrane using a pH-responsive ratiometric AIEgen.Chem Sci. 2020 May 15;11(22):5753-5758. doi: 10.1039/d0sc02097d. eCollection 2020 Jun 14. Chem Sci. 2020. PMID: 32832051 Free PMC article.
-
Assay for evaluation of proarrhythmic effects of herbal products: Case study with 12 Evodia preparations.Toxicol Rep. 2023 Apr 26;10:589-599. doi: 10.1016/j.toxrep.2023.04.014. eCollection 2023. Toxicol Rep. 2023. PMID: 37213814 Free PMC article.
References
-
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440(7083):463–9. doi: 10.1038/nature04710 . - DOI - PubMed
-
- Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92(3):1393–478. . - PubMed
-
- Saxena P, Zangerl-Plessl EM, Linder T, Windisch A, Hohaus A, Timin E, et al. New potential binding determinant for hERG channel inhibitors. Sci Rep. 2016;6:24182 doi: 10.1038/srep24182 ; - DOI - PMC - PubMed
-
- Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 2015;14(7):475–86. doi: 10.1038/nrd4609 . - DOI - PubMed
-
- Kannankeril P, Roden DM, Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62(4):760–81. doi: 10.1124/pr.110.003723 . - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

