Infectious bronchitis corona virus establishes productive infection in avian macrophages interfering with selected antimicrobial functions
- PMID: 28763472
- PMCID: PMC5538654
- DOI: 10.1371/journal.pone.0181801
Infectious bronchitis corona virus establishes productive infection in avian macrophages interfering with selected antimicrobial functions
Abstract
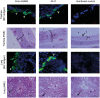
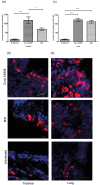
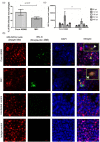
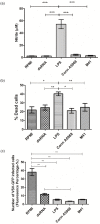
Infectious bronchitis virus (IBV) causes respiratory disease leading to loss of egg and meat production in chickens. Although it is known that macrophage numbers are elevated in the respiratory tract of IBV infected chickens, the role played by macrophages in IBV infection, particularly as a target cell for viral replication, is unknown. In this study, first, we investigated the ability of IBV to establish productive replication in macrophages in lungs and trachea in vivo and in macrophage cell cultures in vitro using two pathogenic IBV strains. Using a double immunofluorescent technique, we observed that both IBV Massachusetts-type 41 (M41) and Connecticut A5968 (Conn A5968) strains replicate in avian macrophages at a low level in vivo. This in vivo observation was substantiated by demonstrating IBV antigens in macrophages following in vitro IBV infection. Further, IBV productive infection in macrophages was confirmed by demonstrating corona viral particles in macrophages and IBV ribonucleic acid (RNA) in culture supernatants. Evaluation of the functions of macrophages following infection of macrophages with IBV M41 and Conn A5968 strains revealed that the production of antimicrobial molecule, nitric oxide (NO) is inhibited. It was also noted that replication of IBV M41 and Conn A5968 strains in macrophages does not interfere with the induction of type 1 IFN activity by macrophages. In conclusion, both M41 and Con A5968 IBV strains infect macrophages in vivo and in vitro resulting productive replications. During the replication of IBV in macrophages, their ability to produce NO can be affected without affecting the ability to induce type 1 IFN activity. Further studies are warranted to uncover the significance of macrophage infection of IBV in the pathogenesis of IBV infection in chickens.
Conflict of interest statement
Figures


References
-
- Montassier HJ. Molecular epidemiology and evolution of avian infectious bronchitis virus. Revista Brasileira de Ciência Avícola. 2010;12:87–96.
-
- Reddy VRAP, Trus I, Desmarets LMB, Li Y, Theuns S, Nauwynck HJ. Productive replication of nephropathogenic infectious bronchitis virus in peripheral blood monocytic cells, a strategy for viral dissemination and kidney infection in chickens. Veterinary Research. 2016;47(1):70 10.1186/s13567-016-0354-9 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

