Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial
- PMID: 28763548
- PMCID: PMC5817603
- DOI: 10.1001/jama.2017.9362
Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial
Abstract
Importance: Guidelines recommend corticosteroids in patients with IgA nephropathy and persistent proteinuria, but the effects remain uncertain.
Objective: To evaluate the efficacy and safety of corticosteroids in patients with IgA nephropathy at risk of progression.
Design, setting, and participants: The Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING) study was a multicenter, double-blind, randomized clinical trial designed to recruit 750 participants with IgA nephropathy (proteinuria greater than 1 g/d and estimated glomerular filtration rate [eGFR] of 20 to 120 mL/min/1.73 m2 after at least 3 months of blood pressure control with renin-angiotensin system blockade] and to provide follow-up until 335 primary outcomes occurred.
Interventions: Patients were randomized 1:1 to oral methylprednisolone (0.6-0.8 mg/kg/d; maximum, 48 mg/d) (n = 136) or matching placebo (n = 126) for 2 months, with subsequent weaning over 4 to 6 months.
Main outcomes and measures: The primary composite outcome was end-stage kidney disease, death due to kidney failure, or a 40% decrease in eGFR. Predefined safety outcomes were serious infection, new diabetes, gastrointestinal hemorrhage, fracture/osteonecrosis, and cardiovascular events. The mean required follow-up was estimated to be 5 years.
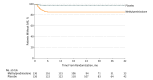
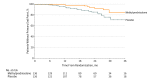
Results: After randomization of 262 participants (mean age, 38.6 [SD, 11.1] years; 96 [37%] women; eGFR, 59.4 mL/min/1.73 m2; urine protein excretion, 2.40 g/d) and 2.1 years' median follow-up, recruitment was discontinued because of excess serious adverse events. Serious events occurred in 20 participants (14.7%) in the methylprednisolone group vs 4 (3.2%) in the placebo group (P = .001; risk difference, 11.5% [95% CI, 4.8%-18.2%]), mostly due to excess serious infections (11 [8.1%] vs 0; risk difference, 8.1% [95% CI, 3.5%-13.9%]; P < .001), including 2 deaths. The primary renal outcome occurred in 8 participants (5.9%) in the methylprednisolone group vs 20 (15.9%) in the placebo group (hazard ratio, 0.37 [95% CI, 0.17-0.85]; risk difference, 10.0% [95% CI, 2.5%-17.9%]; P = .02).
Conclusions and relevance: Among patients with IgA nephropathy and proteinuria of 1 g/d or greater, oral methylprednisolone was associated with an increased risk of serious adverse events, primarily infections. Although the results were consistent with potential renal benefit, definitive conclusions about treatment benefit cannot be made, owing to early termination of the trial.
Trial registration: clinicaltrials.gov Identifier: NCT01560052.
Conflict of interest statement
Figures


Comment in
-
Corticosteroids for IgA Nephropathy: TESTING for Benefit, Discovering Harm.JAMA. 2017 Aug 1;318(5):429-431. doi: 10.1001/jama.2017.9359. JAMA. 2017. PMID: 28763530 No abstract available.
-
In patients with proteinuric IgA nephropathy, benefits of methylprednisolone were offset by harms.Ann Intern Med. 2017 Nov 21;167(10):JC58. doi: 10.7326/ACPJC-2017-167-10-058. Ann Intern Med. 2017. PMID: 29159384 No abstract available.
-
Corticosteroids in IgA Nephropathy.Am J Kidney Dis. 2018 Feb;71(2):160-162. doi: 10.1053/j.ajkd.2017.10.004. Epub 2017 Dec 2. Am J Kidney Dis. 2018. PMID: 29203129 No abstract available.
-
TESTING Corticosteroids in IgA Nephropathy: A Continuing Challenge.Clin J Am Soc Nephrol. 2018 Jan 6;13(1):158-160. doi: 10.2215/CJN.10560917. Epub 2017 Dec 13. Clin J Am Soc Nephrol. 2018. PMID: 29237704 Free PMC article. No abstract available.
References
-
- Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402-2414. - PubMed
-
- Le W, Liang S, Hu Y, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479-1485. - PubMed
-
- Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry . Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177-3183. - PubMed
-
- Lv J, Zhang H, Zhou Y, Li G, Zou W, Wang H. Natural history of immunoglobulin A nephropathy and predictive factors of prognosis: a long-term follow up of 204 cases in China. Nephrology (Carlton). 2008;13(3):242-246. - PubMed
-
- Coppo R, Peruzzi L, Amore A, et al. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007;18(6):1880-1888. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

