Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice
- PMID: 28763559
- PMCID: PMC5539800
- DOI: 10.1167/iovs.17-21796
Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice
Erratum in
-
Erratum in: Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice.Invest Ophthalmol Vis Sci. 2022 Apr 1;63(4):4. doi: 10.1167/iovs.63.4.4. Invest Ophthalmol Vis Sci. 2022. PMID: 35385073 Free PMC article. No abstract available.
Abstract
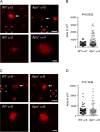
Purpose: Neovascular age-related macular degeneration (AMD) is a major cause of legal blindness in the elderly. Diets with omega3-long-chain-polyunsaturated-fatty-acid (ω3-LCPUFA) correlate with a decreased risk of AMD. Dietary ω3-LCPUFA versus ω6-LCPUFA inhibits mouse ocular neovascularization, but the underlying mechanism needs further exploration. The aim of this study was to investigate if adiponectin (APN) mediated ω3-LCPUFA suppression of neovessels in AMD.
Methods: The mouse laser-induced choroidal neovascularization (CNV) model was used to mimic some of the inflammatory aspect of AMD. CNV was compared between wild-type (WT) and Apn-/- mice fed either otherwise matched diets with 2% ω3 or 2% ω6-LCPUFAs. Vldlr-/- mice were used to mimic some of the metabolic aspects of AMD. Choroid assay ex vivo and human retinal microvascular endothelial cell (HRMEC) proliferation assay in vitro was used to investigate the APN pathway in angiogenesis. Western blot for p-AMPKα/AMPKα and qPCR for Apn, Mmps, and IL-10 were used to define mechanism.
Results: ω3-LCPUFA intake suppressed laser-induced CNV in WT mice; suppression was abolished with APN deficiency. ω3-LCPUFA, mediated by APN, decreased mouse Mmps expression. APN deficiency decreased AMPKα phosphorylation in vivo and exacerbated choroid-sprouting ex vivo. APN pathway activation inhibited HRMEC proliferation and decreased Mmps. In Vldlr-/- mice, ω3-LCPUFA increased retinal AdipoR1 and inhibited NV. ω3-LCPUFA decreased IL-10 but did not affect Mmps in Vldlr-/- retinas.
Conclusions: APN in part mediated ω3-LCPUFA inhibition of neovascularization in two mouse models of AMD. Modulating the APN pathway in conjunction with a ω3-LCPUFA-enriched-diet may augment the beneficial effects of ω3-LCPUFA in AMD patients.
Figures




References
-
- Merle BM, Delyfer MN, Korobelnik JF,et al. . High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J Nutr. 2013; 143: 505– 511. - PubMed
-
- Tan JS, Wang JJ, Flood V, Mitchell P. . Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009; 127: 656– 665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

