β1-Integrin Deletion From the Lens Activates Cellular Stress Responses Leading to Apoptosis and Fibrosis
- PMID: 28763805
- PMCID: PMC5539801
- DOI: 10.1167/iovs.17-21721
β1-Integrin Deletion From the Lens Activates Cellular Stress Responses Leading to Apoptosis and Fibrosis
Abstract
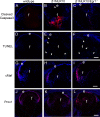
Purpose: Previous research showed that the absence of β1-integrin from the mouse lens after embryonic day (E) 13.5 (β1MLR10) leads to the perinatal apoptosis of lens epithelial cells (LECs) resulting in severe microphthalmia. This study focuses on elucidating the molecular connections between β1-integrin deletion and this phenotype.
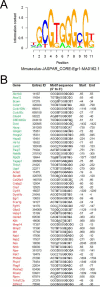
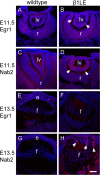
Methods: RNA sequencing was performed to identify differentially regulated genes (DRGs) in β1MLR10 lenses at E15.5. By using bioinformatics analysis and literature searching, Egr1 (early growth response 1) was selected for further study. The activation status of certain signaling pathways (focal adhesion kinase [FAK]/Erk, TGF-β, and Akt signaling) was studied via Western blot and immunohistochemistry. Mice lacking both β1-integrin and Egr1 genes from the lenses were created (β1MLR10/Egr1-/-) to study their relationship.
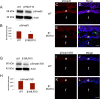
Results: RNA sequencing identified 120 DRGs that include candidates involved in the cellular stress response, fibrosis, and/or apoptosis. Egr1 was investigated in detail, as it mediates cellular stress responses in various cell types, and is recognized as an upstream regulator of numerous other β1MLR10 lens DRGs. In β1MLR10 mice, Egr1 levels are elevated shortly after β1-integrin loss from the lens. Further, pErk1/2 and pAkt are elevated in β1MLR10 LECs, thus providing the potential signaling mechanism that causes Egr1 upregulation in the mutant. Indeed, deletion of Egr1 from β1MLR10 lenses partially rescues the microphthalmia phenotype.
Conclusions: β1-integrin regulates the appropriate levels of Erk1/2 and Akt phosphorylation in LECs, whereas its deficiency results in the overexpression of Egr1, culminating in reduced cell survival. These findings provide insight into the molecular mechanism underlying the microphthalmia observed in β1MLR10 mice.
Figures






Similar articles
-
β1-integrin controls cell fate specification in early lens development.Differentiation. 2016 Oct-Nov;92(4):133-147. doi: 10.1016/j.diff.2016.08.002. Epub 2016 Sep 3. Differentiation. 2016. PMID: 27596755 Free PMC article.
-
Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype.Dev Biol. 2007 Jun 15;306(2):658-68. doi: 10.1016/j.ydbio.2007.04.004. Epub 2007 Apr 12. Dev Biol. 2007. PMID: 17493607 Free PMC article.
-
Integrin beta1-mediated signaling is involved in transforming growth factor-beta2-promoted migration in human lens epithelial cells.Mol Vis. 2007 Sep 20;13:1769-76. Mol Vis. 2007. PMID: 17960115
-
In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated β1 integrin and induction of FAK/PI3K/Akt motility signaling.Oncogene. 2014 Jan 9;33(2):255-68. doi: 10.1038/onc.2012.547. Epub 2012 Dec 3. Oncogene. 2014. PMID: 23208492 Free PMC article.
-
Integrins in lens development and disease.Exp Eye Res. 2009 Feb;88(2):216-25. doi: 10.1016/j.exer.2008.06.020. Epub 2008 Jul 11. Exp Eye Res. 2009. PMID: 18671967 Free PMC article. Review.
Cited by
-
The Immediate Early Response of Lens Epithelial Cells to Lens Injury.Cells. 2022 Nov 1;11(21):3456. doi: 10.3390/cells11213456. Cells. 2022. PMID: 36359852 Free PMC article.
-
Lens Epithelial Cells Initiate an Inflammatory Response Following Cataract Surgery.Invest Ophthalmol Vis Sci. 2018 Oct 1;59(12):4986-4997. doi: 10.1167/iovs.18-25067. Invest Ophthalmol Vis Sci. 2018. PMID: 30326070 Free PMC article.
-
A comprehensive spatial-temporal transcriptomic analysis of differentiating nascent mouse lens epithelial and fiber cells.Exp Eye Res. 2018 Oct;175:56-72. doi: 10.1016/j.exer.2018.06.004. Epub 2018 Jun 5. Exp Eye Res. 2018. PMID: 29883638 Free PMC article.
-
RNA sequencing-based transcriptomic profiles of embryonic lens development for cataract gene discovery.Hum Genet. 2018 Dec;137(11-12):941-954. doi: 10.1007/s00439-018-1958-0. Epub 2018 Nov 11. Hum Genet. 2018. PMID: 30417254 Free PMC article.
-
SUMO1-regulated DBC1 promotes p53-dependent stress-induced apoptosis of lens epithelial cells.Aging (Albany NY). 2023 Sep 7;15(17):8812-8832. doi: 10.18632/aging.205001. Epub 2023 Sep 7. Aging (Albany NY). 2023. PMID: 37683133 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

