Drug survival of second biological DMARD therapy in patients with rheumatoid arthritis: a retrospective non-interventional cohort analysis
- PMID: 28764705
- PMCID: PMC5540414
- DOI: 10.1186/s12891-017-1684-0
Drug survival of second biological DMARD therapy in patients with rheumatoid arthritis: a retrospective non-interventional cohort analysis
Abstract
Background: Since persistence to first biological disease modifying anti-rheumatic drugs (bDMARDs) is far from ideal in rheumatoid arthritis (RA) patients, many do receive a second and/or third bDMARD treatment. However, little is known about treatment persistence of the second-line bDMARD and it is specifically unknown whether the mode of action of such a treatment is associated with different persistence rates. We aimed to assess discontinuation-, re-initiation- or continuation-rates of a 2nd bDMARD therapy as well as switching-rates to a third biological DMARD (3rd bDMARD) therapy in RA patients.
Method: Analysis was based on German claims data (2010-2013). Patients were included if they had received at least one prescription for an anti-TNF and at least one follow-up prescription of a 2nd bDMARD different from the first anti-TNF. Patient follow-up started on the date of the first prescription for the 2nd bDMARD and lasted for 12 months or until a patient's death.
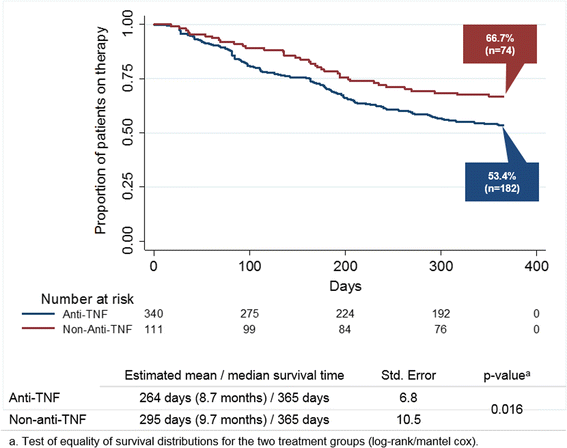
Results: 2667 RA patients received at least one anti-TNF prescription. Of these, 451 patients received a second bDMARD (340 anti-TNF, mean age 52.6 years; 111 non-anti-TNF, mean age 55.9 years). During the follow-up, 28.8% vs. 11.7% of the 2nd anti-TNF vs. non-anti-TNF patients (p < 0.001) switched to a 3rd bDMARD; 14.1% vs. 19.8% (p = 0.179) discontinued without re-start; 3.8% vs.1.8% (p = 0.387) re-started and 53.5 vs. 66.7% (p < 0.050) continued therapy. Patients in the non-anti-TNF group demonstrated longer drug survival (295 days) than patients in the anti-TNF group (264 days; p = 0.016). Independent variables associated with earlier discontinuation (including re-start) or switch were prescription of an anti-TNF as 2nd bDMARD (HR = 1.512) and a higher comorbidity level (CCI, HR = 1.112), whereas previous painkiller medication (HR = 0.629) was associated with later discontinuation or switch.
Conclusions: Only 56.8% of RA patients continued 2nd bDMARD treatment after 12 months; 60% if re-start was included. Non-anti-TNF patients had a higher probability of continuing 2nd bDMARD therapy.
Keywords: Anti-TNF; Continuation of bDMARD therapy; Rheumatoid arthritis; Switch of bDMARD therapy; bDMARD therapy.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
Because of the non-interventional, retrospective nature of this study and because our analysis involved an anonymized dataset, no ethical review was required. This complies with national guidelines and recommendations [34, 35]. Because of the anonymized and retrospective nature of the data, an informed consent was not obtained from the patients.
Consent for publication
Not applicable.
Competing interests
TW has received honoraria from several pharmaceutical/consultancy companies (Novo Nordisk, Abbvie; Merck; GSK, BMS, LEO Pharma, Astra Zeneca, Bayer, Boehringer Ingelheim, Pharmerit). SM and SCL participated in this study as staff members of IPAM; IPAM work in this study was sponsored by BMS. MH and IM participated in this study as staff members of Pharmerit; Pharmerit work in this study was also sponsored by BMS.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Humphreys JH, Verstappen SMM, Hyrich KL, Chipping JR, Marshall T, Symmons DPM. The incidence of rheumatoid arthritis in the UK: comparisons using the 2010 ACR/EULAR classification criteria and the 1987 ACR classification criteria. Results from the Norfolk arthritis register. Ann Rheum Dis. 2013;72:1315–1320. doi: 10.1136/annrheumdis-2012-201960. - DOI - PMC - PubMed
-
- Jani M, Chinoy H, Warren RB, Griffiths CEM, Plant D, Fu B, et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis & Rheumatology. 2015;67:2011–2019. doi: 10.1002/art.39169. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

