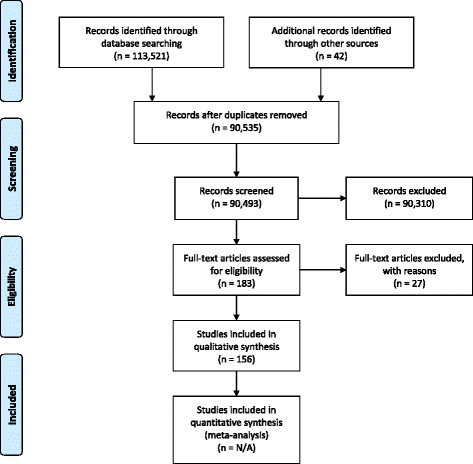
Barriers to the conduct of randomised clinical trials within all disease areas
- PMID: 28764809
- PMCID: PMC5539637
- DOI: 10.1186/s13063-017-2099-9
Barriers to the conduct of randomised clinical trials within all disease areas
Abstract
Background: Randomised clinical trials are key to advancing medical knowledge and to enhancing patient care, but major barriers to their conduct exist. The present paper presents some of these barriers.
Methods: We performed systematic literature searches and internal European Clinical Research Infrastructure Network (ECRIN) communications during face-to-face meetings and telephone conferences from 2013 to 2017 within the context of the ECRIN Integrating Activity (ECRIN-IA) project.
Results: The following barriers to randomised clinical trials were identified: inadequate knowledge of clinical research and trial methodology; lack of funding; excessive monitoring; restrictive privacy law and lack of transparency; complex regulatory requirements; and inadequate infrastructures. There is a need for more pragmatic randomised clinical trials conducted with low risks of systematic and random errors, and multinational cooperation is essential.
Conclusions: The present paper presents major barriers to randomised clinical trials. It also underlines the value of using a pan-European-distributed infrastructure to help investigators overcome barriers for multi-country trials in any disease area.
Keywords: Barriers; Bottlenecks; Challenges; Evidence based clinical practice; Evidence based medicine; Hindrances; Randomised clinical trials.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare apart from their involvement in clinical trials as well as in ECRIN.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- National Institute of Health . NIH inventory of clinical trials: fiscal year 1979, vol. I. Bethesda: Division of Research Grants, Research Analysis and Evaluation Branch; 1979.
-
- Jakobsen JC, Gluud C. The necessity of randomized clinical trials. Br J Med Res. 2013;3(4):i453–1468.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources