Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009-2012
- PMID: 28765084
- PMCID: PMC5591775
- DOI: 10.1016/j.ypmed.2017.07.027
Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009-2012
Abstract
Surveillance of cervical intraepithelial neoplasia grade III (CIN III) and adenocarcinoma in situ (AIS) is important for determining the burden of a preventable disease, identifying effects of vaccination on future diagnoses, and developing targeted programs. We analyzed population-based rates of high-grade cervical cancer precursor lesions using data from four central cancer registries (diagnosis years 2009-2012 from Louisiana, Kentucky, Michigan, and diagnosis years 2011-2012 from Los Angeles) by age, race, and histology. We also compared rates of precursors to invasive cancers. With 4 complete years of data from Michigan, we were able to conduct a trend analysis for that state. Data analysis was conducted in Atlanta during 2016. Kentucky reported the highest rate of CIN III/AIS (69.8), followed by Michigan (55.4), Louisiana (42.3), and Los Angeles (19.2). CIN III/AIS rates declined among women in Michigan by 37% each year for women aged 15-19, 14% for those aged 20-24, and 7% for those aged 25-29. Rates of CIN III/AIS vary by registry, and were higher than invasive cancer. In Michigan, declines in CIN III/AIS among women aged 15-29 are likely related in part to updated screening recommendations, and to the impact of human papillomavirus vaccination.
Keywords: Cervical cancer; Cervical intraepithelial neoplasia; HPV; HPV vaccines; Population-based cancer registries.
Published by Elsevier Inc.
Conflict of interest statement
Figures

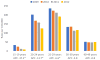
References
-
- [North] American Association of Central Cancer Registries. Working group on pre-invasive cervical neoplasia and population-based cancer registries: final subcommittee report. [N]AACCR conference; 5–6 Apr 1993; Rockville, MD. Subsequently adopted by the [N]AACCR Executive Board May1993 and amended November 1993.
-
- The Bethesda System for reporting cervical/vaginal cytologic diagnoses. Report of the 1991 Bethesda Workshop. Am J Surg Pathol. 1992;16(9):914–6. - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, et al. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2007;56(RR-2):1–24. - PubMed
-
- Petrosky E, Bocchini JA, Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morbidity and mortality weekly report. 2015;64(11):300–4. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

