Discontinuation and non-publication of randomised clinical trials supported by the main public funding body in Switzerland: a retrospective cohort study
- PMID: 28765131
- PMCID: PMC5642669
- DOI: 10.1136/bmjopen-2017-016216
Discontinuation and non-publication of randomised clinical trials supported by the main public funding body in Switzerland: a retrospective cohort study
Abstract
Objective: The Swiss National Science Foundation (SNSF) promotes academic excellence through competitive selection of study proposals and rigorous evaluation of feasibility, but completion status and publication history of SNSF-supported randomised clinical trials (RCTs) remain unclear. The main objectives were to review all healthcare RCTs supported by the SNSF for trial discontinuation and non-publication, to investigate potential risk factors for trial discontinuation due to poor recruitment and non-publication, and to compare findings to other Swiss RCTs not supported by the SNSF.
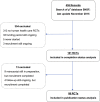
Design: We established a retrospective cohort of all SNSF-supported RCTs for which recruitment and funding had ended in 2015 or earlier. For each RCT, two investigators independently searched corresponding publications in electronic databases. In addition, we approached all principal investigators to ask for additional publications and information about trial discontinuation. Teams of two investigators independently extracted details about study design, recruitment of participants, outcomes, analysis and sample size from the original proposal and, if available, from trial registries and publications. We used multivariable regression analysis to explore potential risk factors associated with discontinuation due to poor recruitment and with non-publication, and to compare our results with data from a previous cohort of Swiss RCTs not supported by the SNSF.
Results: We included 101 RCTs supported by the SNSF between 1986 and 2015. Eighty-seven (86%) principal investigators responded to our survey. Overall, 69 (68%) RCTs were completed, 26 (26%) RCTs were prematurely discontinued (all due to slow recruitment) and the completion status remained unclear for 6 (6%) RCTs. For analysing publication status, we excluded 4 RCTs for which follow-up was still ongoing and 9 for which manuscripts were still in preparation. Of the remaining 88 RCTs, 53 (60%) were published as full articles in peer-reviewed journals. Multivariable regression models suggested that discontinued trials were at higher risk for non-publication than completed trials (adjusted OR 7.61; 95% CI 2.44 to 27.09). Compared with other Swiss RCTs, the risk of discontinuation for SNSF-supported RCTs was higher than in industry-initiated RCTs (adjusted OR 3.84; 95% CI 1.68 to 8.74), but not significantly different from investigator-initiated RCTs not supported by the SNSF (adjusted OR 1.05; 95% CI 0.51 to 2.11). We found no evidence that the proportion of discontinued or unpublished RCTs decreased over the last 20 years.
Conclusions: One out of four SNSF-supported RCTs were prematurely discontinued due to slow recruitment, 40% of all included RCTs and 70% of all discontinued RCTs were not published in peer-reviewed journals. There is a case to reconsider how public funding bodies such as the SNSF could improve their feasibility assessment and promote publication of RCTs irrespective of completion status.
Keywords: early termination of clinical trials; non-publication; randomized controlled trial; swiss national science foundation.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: The study was funded by the Swiss National Science Foundation (SNSF) and the SNSF provided original proposals of SNSF-supported randomized clinical trials, but the SNSF had no role in the design of the study, data extraction, data analysis, interpretation of the results and writing the manuscript. No other financial relationships with any other organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work to declare. There are no conflicts of interest to declare.
Figures
References
-
- Ann B. Research Methods In Health: Investigating Health And Health Services. UK: McGraw-Hill Education, 2014.
-
- Beitragsreglement E - definitive Version 2015_mit_Inhaltsverzeichnis - allg_reglement_16_e.pdf. http://www.snf.ch/SiteCollectionDocuments/allg_reglement_16_e.pdf (accessed 21 Jul 2016).
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources