Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts
- PMID: 28765140
- PMCID: PMC5582407
- DOI: 10.15252/emmm.201607485
Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts
Abstract
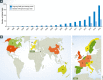
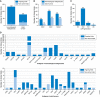
Chimeric antigen receptor (CAR) T cell therapy, together with checkpoint inhibition, has been celebrated as a breakthrough technology due to the substantial benefit observed in clinical trials with patients suffering from relapsed or refractory B-cell malignancies. In this review, we provide a comprehensive overview of the clinical trials performed so far worldwide and analyze parameters such as targeted antigen and indication, CAR molecular design, CAR T cell manufacturing, anti-tumor activities, and related toxicities. More than 200 CAR T cell clinical trials have been initiated so far, most of which aim to treat lymphoma or leukemia patients using CD19-specific CARs. An increasing number of studies address solid tumors as well. Notably, not all clinical trials conducted so far have shown promising results. Indeed, in a few patients CAR T cell therapy resulted in severe adverse events with fatal outcome. Of note, less than 10% of the ongoing CAR T cell clinical trials are performed in Europe. Taking lead from our analysis, we discuss the problems and general hurdles preventing efficient clinical development of CAR T cells as well as opportunities, with a special focus on the European stage.
Keywords: ATMPs; cancer; immunotherapy; regulatory issues; toxicities.
© 2017 The Authors. Published under the terms of the CC BY 4.0 license.
Figures




References
-
- Abou‐El‐Enein M, Schneider CK (2016) Deciphering the EU clinical trials regulation. Nat Biotechnol 34: 231–233 - PubMed
-
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A et al (2015) Human epidermal growth factor receptor 2 (HER2) ‐specific chimeric antigen receptor‐modified T cells for the immunotherapy of HER2‐positive sarcoma. J Clin Oncol 33: 1688–1696 - PMC - PubMed
-
- Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez‐Ojeda O et al (2011) Safety and persistence of adoptively transferred autologous CD19‐targeted T cells in patients with relapsed or chemotherapy refractory B‐cell leukemias. Blood 118: 4817–4828 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

