Non-peptide guided auto-secretion of recombinant proteins by super-folder green fluorescent protein in Escherichia coli
- PMID: 28765554
- PMCID: PMC5539203
- DOI: 10.1038/s41598-017-07421-3
Non-peptide guided auto-secretion of recombinant proteins by super-folder green fluorescent protein in Escherichia coli
Abstract
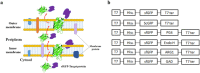
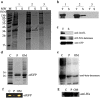
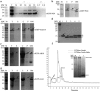
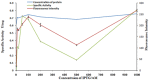
Protein secretion in Escherichia coli is usually led by a signal peptide that targets the protein to specific secretory pathways. In this study, we demonstrated that the superfolder green fluorescent protein (sfGFP) could be served as a non-signal peptide to guide protein auto-secretion in E. coli. This auto-secretion was characterized as a three-step process through the sub-cellular localization analysis: inner membrane trans-location followed by anchoring at outer membrane, and then being released into culture media. We further determined that the beta-barrel structure and net negative charges of sfGFP played important roles in its auto-extracellular secretion property. Using sfGFP as a carrier, heterologous proteins ranging from peptide to complex protein, including antibacterial peptide PG4, endo-beta-N-acethylglucosamindase H (Endo H), human arginase-1 (ARG1), and glutamate decarboxylase (GAD) were all successfully expressed and secreted extracellularly when fused to the carboxyl end of sfGFP. Besides facilitating the extracellular secretion, sfGFP fusion proteins can also be correctly folded and formed the active complex protein structure, including the trimetric human ARG1 and homo-hexametric GAD. This is the first report that sfGFP can guide the secretion of recombinant proteins out of the cells from cytoplasm in E. coli without affecting their conformation and function.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Soluble expression of single-chain variable fragment (scFv) in Escherichia coli using superfolder green fluorescent protein as fusion partner.Appl Microbiol Biotechnol. 2019 Aug;103(15):6071-6079. doi: 10.1007/s00253-019-09925-6. Epub 2019 Jun 7. Appl Microbiol Biotechnol. 2019. PMID: 31175428
-
Expression in E. coli and purification of the fibrillogenic fusion proteins TTR-sfGFP and β2M-sfGFP.Prep Biochem Biotechnol. 2011;41(4):337-49. doi: 10.1080/10826068.2010.548433. Prep Biochem Biotechnol. 2011. PMID: 21967335
-
Translocation of green fluorescent protein by comparative analysis with multiple signal peptides.Biotechnol J. 2012 May;7(5):667-76. doi: 10.1002/biot.201100158. Epub 2011 Sep 20. Biotechnol J. 2012. PMID: 21834133
-
The Carboxy-Terminal Region of Flavobacterium johnsoniae SprB Facilitates Its Secretion by the Type IX Secretion System and Propulsion by the Gliding Motility Machinery.J Bacteriol. 2019 Sep 6;201(19):e00218-19. doi: 10.1128/JB.00218-19. Print 2019 Oct 1. J Bacteriol. 2019. PMID: 31262839 Free PMC article.
-
A Whole-Process Visible Strategy for the Preparation of Rhizomucor miehei Lipase with Escherichia coli Secretion Expression System and the Immobilization.Microb Cell Fact. 2024 May 27;23(1):155. doi: 10.1186/s12934-024-02432-y. Microb Cell Fact. 2024. PMID: 38802857 Free PMC article.
Cited by
-
Biological Function of Prophage-Related Gene Cluster ΔVpaChn25_RS25055~ΔVpaChn25_0714 of Vibrio parahaemolyticus CHN25.Int J Mol Sci. 2024 Jan 23;25(3):1393. doi: 10.3390/ijms25031393. Int J Mol Sci. 2024. PMID: 38338671 Free PMC article.
-
Aliivibrio wodanis as a production host: development of genetic tools for expression of cold-active enzymes.Microb Cell Fact. 2019 Nov 11;18(1):197. doi: 10.1186/s12934-019-1247-1. Microb Cell Fact. 2019. PMID: 31711487 Free PMC article.
-
Visual and High-Efficiency Secretion of SARS-CoV-2 Nanobodies with Escherichia coli.Biomolecules. 2025 Jan 12;15(1):111. doi: 10.3390/biom15010111. Biomolecules. 2025. PMID: 39858505 Free PMC article.
-
Toward Light-Regulated Living Biomaterials.Adv Sci (Weinh). 2018 Jun 29;5(8):1800383. doi: 10.1002/advs.201800383. eCollection 2018 Aug. Adv Sci (Weinh). 2018. PMID: 30128245 Free PMC article.
-
Whole-Cell Display of Phospholipase D in Escherichia coli for High-Efficiency Extracellular Phosphatidylserine Production.Biomolecules. 2024 Apr 2;14(4):430. doi: 10.3390/biom14040430. Biomolecules. 2024. PMID: 38672447 Free PMC article.
References
-
- Choi JH, Keum KC, Lee SY. Production of recombinant proteins by high cell density culture of Escherichia coli. Chem. Eng. Sci. 2006;61:876–885. doi: 10.1016/j.ces.2005.03.031. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

