2-Oxoesters: A Novel Class of Potent and Selective Inhibitors of Cytosolic Group IVA Phospholipase A2
- PMID: 28765606
- PMCID: PMC5539244
- DOI: 10.1038/s41598-017-07330-5
2-Oxoesters: A Novel Class of Potent and Selective Inhibitors of Cytosolic Group IVA Phospholipase A2
Abstract
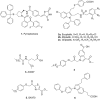
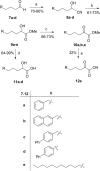
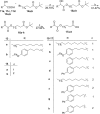
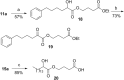
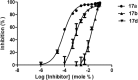
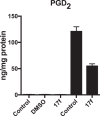
Cytosolic phospholipase A2 (GIVA cPLA2) is the only PLA2 that exhibits a marked preference for hydrolysis of arachidonic acid containing phospholipid substrates releasing free arachidonic acid and lysophospholipids and giving rise to the generation of diverse lipid mediators involved in inflammatory conditions. Thus, the development of potent and selective GIVA cPLA2 inhibitors is of great importance. We have developed a novel class of such inhibitors based on the 2-oxoester functionality. This functionality in combination with a long aliphatic chain or a chain carrying an appropriate aromatic system, such as the biphenyl system, and a free carboxyl group leads to highly potent and selective GIVA cPLA2 inhibitors (X I(50) values 0.00007-0.00008) and docking studies aid in understanding this selectivity. A methyl 2-oxoester, with a short chain carrying a naphthalene ring, was found to preferentially inhibit the other major intracellular PLA2, the calcium-independent PLA2. In RAW264.7 macrophages, treatment with the most potent 2-oxoester GIVA cPLA2 inhibitor resulted in over 50% decrease in KLA-elicited prostaglandin D2 production. The novel, highly potent and selective GIVA cPLA2 inhibitors provide excellent tools for the study of the role of the enzyme and could contribute to the development of novel therapeutic agents for the treatment of inflammatory diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

