Expanded Gene Panel Use for Women With Breast Cancer: Identification and Intervention Beyond Breast Cancer Risk
- PMID: 28766213
- PMCID: PMC5594040
- DOI: 10.1245/s10434-017-5963-7
Expanded Gene Panel Use for Women With Breast Cancer: Identification and Intervention Beyond Breast Cancer Risk
Abstract
Background: Clinicians ordering multi-gene next-generation sequencing panels for hereditary breast cancer risk have a variety of test panel options. Many panels include lesser known breast cancer genes or genes associated with other cancers. The authors hypothesized that using broader gene panels increases the identification of clinically significant findings, some relevant and others incidental to the testing indication. They examined clinician ordering patterns and compared the yield of pathogenic or likely pathogenic (P/LP) variants in non-BRCA genes of female breast cancer patients.
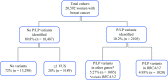
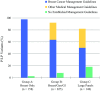
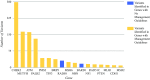
Methods: This study analyzed de-identified personal and family histories in 1085 breast cancer cases with P/LP multi-gene panel findings in non-BRCA cancer genes and sorted them into three groups by the panel used for testing: group A (breast cancer genes only), group B (commonly assessed cancers: breast, gynecologic, and gastrointestinal), and group C (a more expanded set of tumors). The frequency of P/LP variants in genes with established management guidelines was compared and evaluated for consistency with personal and family histories.
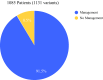
Results: This study identified 1131 P/LP variants and compared variants in clinically actionable genes for breast and non-breast cancers. Overall, 91.5% of these variants were in genes with management guidelines. Nearly 12% were unrelated to personal or family history.
Conclusion: Broader panels were used for 85.6% of our cohort (groups B and C). Although pathogenic variants in non-BRCA genes are reportedly rare, the study found that most were in clinically actionable genes. Expanded panel testing improved the identification of hereditary cancer risk. Small, breast-limited panels may miss clinically relevant findings in genes associated with other heritable cancers.
Figures
References
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Genetic/familial high-risk assessment: breast and ovarian. Version 2.2017. https://www.nccn.org/. Accessed 31 March 2017.
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Genetic/familial high-risk assessment: colorectal. Version 2.2016. https://www.nccn.org/. Accessed 31 March 2017.
-
- The American Society of Breast Surgeons Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer (revised March 14, 2017). https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statement.... Accessed 1 April 2017.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

