Interventions for emergency contraception
- PMID: 28766313
- PMCID: PMC6483633
- DOI: 10.1002/14651858.CD001324.pub5
Interventions for emergency contraception
Update in
-
Interventions for emergency contraception.Cochrane Database Syst Rev. 2019 Jan 20;1(1):CD001324. doi: 10.1002/14651858.CD001324.pub6. Cochrane Database Syst Rev. 2019. PMID: 30661244 Free PMC article.
Abstract
Background: Emergency contraception (EC) is using a drug or copper intrauterine device (Cu-IUD) to prevent pregnancy shortly after unprotected intercourse. Several interventions are available for EC. Information on the comparative effectiveness, safety and convenience of these methods is crucial for reproductive healthcare providers and the women they serve. This is an update of a review previously published in 2009 and 2012.
Objectives: To determine which EC method following unprotected intercourse is the most effective, safe and convenient to prevent pregnancy.
Search methods: In February 2017 we searched CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, Popline and PubMed, The Chinese biomedical databases and UNDP/UNFPA/WHO/World Bank Special Programme on Human Reproduction (HRP) emergency contraception database. We also searched ICTRP and ClinicalTrials.gov as well as contacting content experts and pharmaceutical companies, and searching reference lists of appropriate papers.
Selection criteria: Randomised controlled trials including women attending services for EC following a single act of unprotected intercourse were eligible.
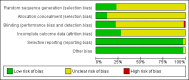
Data collection and analysis: We used standard methodological procedures recommended by Cochrane. The primary review outcome was observed number of pregnancies. Side effects and changes of menses were secondary outcomes.
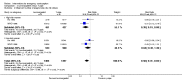
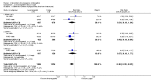
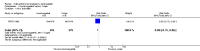
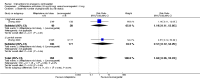
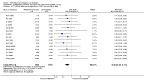
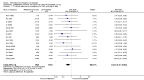
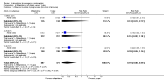
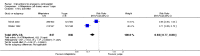
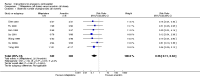
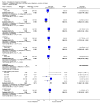
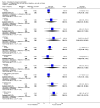
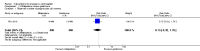
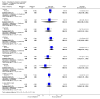
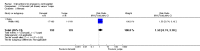
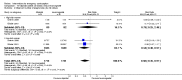
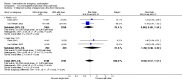
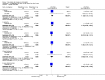
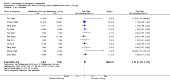
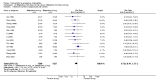
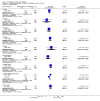
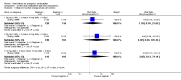
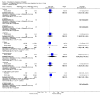
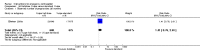
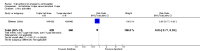
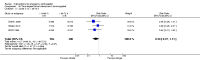
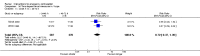
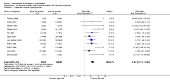
Main results: We included 115 trials with 60,479 women in this review. The quality of the evidence for the primary outcome ranged from moderate to high, and for other outcomes ranged from very low to high. The main limitations were risk of bias (associated with poor reporting of methods), imprecision and inconsistency. Comparative effectiveness of different emergency contraceptive pills (ECP)Levonorgestrel was associated with fewer pregnancies than Yuzpe (estradiol-levonorgestrel combination) (RR 0.57, 95% CI 0.39 to 0.84, 6 RCTs, n = 4750, I2 = 23%, high-quality evidence). This suggests that if the chance of pregnancy using Yuzpe is assumed to be 29 women per 1000, the chance of pregnancy using levonorgestrel would be between 11 and 24 women per 1000.Mifepristone (all doses) was associated with fewer pregnancies than Yuzpe (RR 0.14, 95% CI 0.05 to 0.41, 3 RCTs, n = 2144, I2 = 0%, high-quality evidence). This suggests that if the chance of pregnancy following Yuzpe is assumed to be 25 women per 1000 women, the chance following mifepristone would be between 1 and 10 women per 1000.Both low-dose mifepristone (less than 25 mg) and mid-dose mifepristone (25 mg to 50 mg) were probably associated with fewer pregnancies than levonorgestrel (RR 0.72, 95% CI 0.52 to 0.99, 14 RCTs, n = 8752, I2 = 0%, high-quality evidence; RR 0.61, 95% CI 0.45 to 0.83, 27 RCTs, n = 6052, I2 = 0%, moderate-quality evidence; respectively). This suggests that if the chance of pregnancy following levonorgestrel is assumed to be 20 women per 1000, the chance of pregnancy following low-dose mifepristone would be between 10 and 20 women per 1000; and that if the chance of pregnancy following levonorgestrel is assumed to be 35 women per 1000, the chance of pregnancy following mid-dose mifepristone would be between 16 and 29 women per 1000.Ulipristal acetate (UPA) was associated with fewer pregnancies than levonorgestrel (RR 0.59; 95% CI 0.35 to 0.99, 2 RCTs, n = 3448, I2 = 0%, high-quality evidence). Comparative effectiveness of different ECP dosesIt was unclear whether there was any difference in pregnancy rate between single-dose levonorgestrel (1.5 mg) and the standard two-dose regimen (0.75 mg 12 hours apart) (RR 0.84, 95% CI 0.53 to 1.33, 3 RCTs, n = 6653, I2 = 0%, moderate-quality evidence).Mid-dose mifepristone was associated with fewer pregnancies than low-dose mifepristone (RR 0.73; 95% CI 0.55 to 0.97, 25 RCTs, n = 11,914, I2 = 0%, high-quality evidence). Comparative effectiveness of Cu-IUD versus mifepristoneThere was no conclusive evidence of a difference in the risk of pregnancy between the Cu-IUD and mifepristone (RR 0.33, 95% CI 0.04 to 2.74, 2 RCTs, n = 395, low-quality evidence). Adverse effectsNausea and vomiting were the main adverse effects associated with emergency contraception. There is probably a lower risk of nausea (RR 0.63, 95% CI 0.53 to 0.76, 3 RCTs, n = 2186 , I2 = 59%, moderate-quality evidence) or vomiting (RR 0.12, 95% CI 0.07 to 0.20, 3 RCTs, n = 2186, I2 = 0%, high-quality evidence) associated with mifepristone than with Yuzpe. levonorgestrel is probably associated with a lower risk of nausea (RR 0.40, 95% CI 0.36 to 0.44, 6 RCTs, n = 4750, I2 = 82%, moderate-quality evidence), or vomiting (RR 0.29, 95% CI 0.24 to 0.35, 5 RCTs, n = 3640, I2 = 78%, moderate-quality evidence) than Yuzpe. Levonorgestrel users were less likely to have any side effects than Yuzpe users (RR 0.80, 95% CI 0.75 to 0.86; 1 RCT, n = 1955, high-quality evidence). UPA users were more likely than levonorgestrel users to have resumption of menstruation after the expected date (RR 1.65, 95% CI 1.42 to 1.92, 2 RCTs, n = 3593, I2 = 0%, high-quality evidence). Menstrual delay was more common with mifepristone than with any other intervention and appeared to be dose-related. Cu-IUD may be associated with higher risks of abdominal pain than mifepristone (18 events in 95 women using Cu-IUD versus no events in 190 women using mifepristone, low-quality evidence).
Authors' conclusions: Levonorgestrel and mid-dose mifepristone (25 mg to 50 mg) were more effective than Yuzpe regimen. Both mid-dose (25 mg to 50 mg) and low-dose mifepristone(less than 25 mg) were probably more effective than levonorgestrel (1.5 mg). Mifepristone low dose (less than 25 mg) was less effective than mid-dose mifepristone. UPA was more effective than levonorgestrel.Levonorgestrel users had fewer side effects than Yuzpe users, and appeared to be more likely to have a menstrual return before the expected date. UPA users were probably more likely to have a menstrual return after the expected date. Menstrual delay was probably the main adverse effect of mifepristone and seemed to be dose-related. Cu-IUD may be associated with higher risks of abdominal pain than ECPs.
Conflict of interest statement
JS: no conflicts of interest. YC: no conflicts of interest KC: no conflicts of interest LC: participated in emergency contraceptive trials included in this review (including Chen 2011). LC did not participate in selecting these studies, or extracting their data. LC has received travel costs and consulting fees from Regenex Pharmaceutical Corporation, China Resources Zizhu Pharmaceutical Co, and Bayer Pharma AG. ES: no conflicts of interest
Figures





































































































Update of
-
Interventions for emergency contraception.Cochrane Database Syst Rev. 2012 Aug 15;(8):CD001324. doi: 10.1002/14651858.CD001324.pub4. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Aug 02;8:CD001324. doi: 10.1002/14651858.CD001324.pub5. PMID: 22895920 Updated.
References
References to studies included in this review
-
- Arowojolu AO, Okewole LA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception 2002;66:269‐73. - PubMed
-
- Ashok PW, Stalder C, Wagaarachchi PT, Flett GM, Melvin L, Templeton A. A randomised study comparing a low dose of mifepristone and the Yuzpe regimen for emergency contraception. BJOG 2002;109:553‐60. - PubMed
-
- Askalani AH, Al‐Senity AM, Al‐Agizy HM, Salam HI, Al‐Masry GI, El‐Sadek SM. Evaluation of copper T‐200 as a post‐coital contraceptive. Egyptian Journal of Obstetrics and Gynaecology 1987;13:63‐6.
-
- Bu GY, Yang MY, Cao XL. Clinical study on administration of low dose of mifepristone and levonorgestrel in urgent postcoital contraception [小剂量米非司酮和左炔诺孕酮用于紧急避孕的临床研究]. Progress in Modern Biomedicine 2006;6(2):53‐4.
-
- Cao P, Li M, Xu J, Li Q. Different doses of mifepristone for emergency contraception [不同剂量米非司酮用于紧急避孕效果的观察与分析]. Journal of Practical Obstetrics and Gynecology 1999;15:295‐6.
References to studies excluded from this review
-
- Ashok PW, Wagaarachchi PT, Flett GM, Templeton A. Mifepristone as a late post‐coital contraceptive. Human Reproduction 2001;16:72‐5. - PubMed
-
- Ashok PW, Hamoda H, Flett GMM, Templeton A. Mifepristone versus the Yuzpe regimen (PC4) for emergency contraception. International Journal of Gynecology and Obstetrics 2004;87:188‐93. - PubMed
-
- Ban X, Xiao Y, Fan H, Liu G, Liu Q, Yu L. A comparative clinical study on Tcu380A and Cu‐IUD for emergency contraception. Maternal & Child Health Care of China 2001;16:498‐501.
-
- Benagiano G, Hertzen H. Towards more effective emergency contraception?. Lancet 2010;375(9714):527‐28. - PubMed
-
- Brache V, Cochon L, Deniaud M, Croxatto HB. Ulipristal acetate prevents ovulation more effectively than levonorgestral: analysis of pooled data from three randomized trials of emergency contraception regimens. Contraception 2013;88(5):611‐8. - PubMed
References to ongoing studies
-
- NCT01539720. Levonorgestrel intrauterine system for emergency contraception (LIFE). clinicaltrials.gov/show/NCT01539720 first received: 21 February, 2012.
-
- NCT02175030. RAPID EC ‐ RCT assessing pregnancy with intrauterine devices for EC. clinicaltrials.gov/show/NCT02175030 first received: 23 June , 2014.
-
- NCT02577601. Impact of combined hormonal contraceptives on UPA. clinicaltrials.gov/show/NCT02577601 first received: 5 August , 2015.
Additional references
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
-
- Fine P, Mathe H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception.. Obstet Gynecol 2010;115:257‐63. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

