Acceleration Mechanisms of Skin Wound Healing by Autologous Micrograft in Mice
- PMID: 28767054
- PMCID: PMC5578065
- DOI: 10.3390/ijms18081675
Acceleration Mechanisms of Skin Wound Healing by Autologous Micrograft in Mice
Abstract
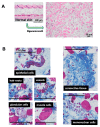
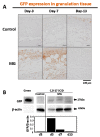
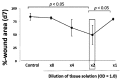
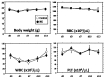
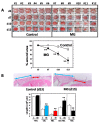
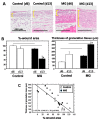
A micrograft technique, which minces tissue into micro-fragments >50 μm, has been recently developed. However, its pathophysiological mechanisms in wound healing are unclear yet. We thus performed a wound healing study using normal mice. A humanized mouse model of a skin wound with a splint was used. After total skin excision, tissue micro-fragments obtained by the Rigenera protocol were infused onto the wounds. In the cell tracing study, GFP-expressing green mice and SCID mice were used. Collagen stains including Picrosirius red (PSR) and immunohistological stains for α-smooth muscle actin (αSMA), CD31, transforming growth factor-β1 (TGF-β1) and neutrophils were evaluated for granulation tissue development. GFP-positive cells remained in granulation tissue seven days after infusion, but vanished after 13 days. Following the infusion of the tissue micrograft solution onto the wound, TGF-β1 expression was transiently upregulated in granulation tissue in the early phase. Subsequently, αSMA-expressing myofibroblasts increased in number in thickened granulation tissue with acceleration of neovascularization and collagen matrix maturation. On such granulation tissue, regenerative epithelial healing progressed, resulting in wound area reduction. Alternative alteration after the micrograft may have increased αSMA-expressing myofibroblasts in granulation tissue, which may act on collagen accumulation, neovascularization and wound contraction. All of these changes are favorable for epithelial regeneration on wound.
Keywords: TGF-β1; granulation tissue; micrograft; wound healing; αSMA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

