Intramitochondrial Ascorbic Acid Enhances the Formation of Mitochondrial Superoxide Induced by Peroxynitrite via a Ca2+-Independent Mechanism
- PMID: 28767071
- PMCID: PMC5578076
- DOI: 10.3390/ijms18081686
Intramitochondrial Ascorbic Acid Enhances the Formation of Mitochondrial Superoxide Induced by Peroxynitrite via a Ca2+-Independent Mechanism
Abstract
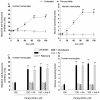
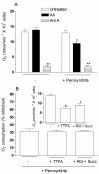
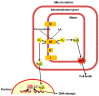
Exposure of U937 cells to peroxynitrite promotes mitochondrial superoxide formation via a mechanism dependent on both inhibition of complex III and increased mitochondrial Ca2+ accumulation. Otherwise inactive concentrations of the oxidant produced the same maximal effects in the presence of either complex III inhibitors or agents mobilizing Ca2+ from the ryanodine receptor and enforcing its mitochondrial accumulation. l-Ascorbic acid (AA) produced similar enhancing effects in terms of superoxide formation, DNA strand scission and cytotoxicity. However, AA failed to enhance the intra-mitochondrial concentration of Ca2+ and the effects observed in cells supplemented with peroxinitrite, while insensitive to manipulations preventing the mobilization of Ca2+, or the mitochondrial accumulation of the cation, were also detected in human monocytes and macrophages, which do not express the ryanodine receptor. In all these cell types, mitochondrial permeability transition-dependent toxicity was detected in cells exposed to AA/peroxynitrite and, based on the above criteria, these responses also appeared Ca2+-independent. The enhancing effects of AA are therefore similar to those mediated by bona fide complex III inhibitors, although the vitamin failed to directly inhibit complex III, and in fact enhanced its sensitivity to the inhibitory effects of peroxynitrite.
Keywords: DNA damage; ascorbic acid; complex III; mitochondrial dysfunction; mitochondrial superoxide; peroxynitrite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
A novel mechanism, uniquely dependent on mitochondrial calcium accumulation, whereby peroxynitrite promotes formation of superoxide/hydrogen peroxide and the ensuing strand scission of genomic DNA.Antioxid Redox Signal. 2010 Sep 15;13(6):745-56. doi: 10.1089/ars.2009.2845. Antioxid Redox Signal. 2010. PMID: 20136509
-
Inhibition of complex III promotes loss of Ca2+ dependence for mitochondrial superoxide formation and permeability transition evoked by peroxynitrite.J Cell Sci. 2007 Jun 1;120(Pt 11):1908-14. doi: 10.1242/jcs.003228. Epub 2007 May 15. J Cell Sci. 2007. PMID: 17504811
-
Mitochondrial ascorbic acid is responsible for enhanced susceptibility of U937 cells to the toxic effects of peroxynitrite.Biofactors. 2014 Mar-Apr;40(2):236-46. doi: 10.1002/biof.1139. Epub 2013 Sep 16. Biofactors. 2014. PMID: 24105898
-
Peroxynitrite damages U937 cell DNA via the intermediate formation of mitochondrial oxidants.IUBMB Life. 2008 Nov;60(11):753-6. doi: 10.1002/iub.116. IUBMB Life. 2008. PMID: 18642347 Review.
-
Survival pathways triggered by peroxynitrite in cells belonging to the monocyte/macrophage lineage.Comp Biochem Physiol A Mol Integr Physiol. 2005 Oct;142(2):118-23. doi: 10.1016/j.cbpb.2005.05.037. Epub 2005 Jun 17. Comp Biochem Physiol A Mol Integr Physiol. 2005. PMID: 15964776 Review.
Cited by
-
Selective Targeting of Cancerous Mitochondria and Suppression of Tumor Growth Using Redox-Active Treatment Adjuvant.Oxid Med Cell Longev. 2020 Nov 2;2020:6212935. doi: 10.1155/2020/6212935. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33204397 Free PMC article.
-
Doxycycline, Azithromycin and Vitamin C (DAV): A potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs).Aging (Albany NY). 2019 Apr 19;11(8):2202-2216. doi: 10.18632/aging.101905. Aging (Albany NY). 2019. PMID: 31002656 Free PMC article.
-
Differentiation of Promonocytic U937 Cells to Monocytes Is Associated with Reduced Mitochondrial Transport of Ascorbic Acid.Oxid Med Cell Longev. 2018 Feb 8;2018:4194502. doi: 10.1155/2018/4194502. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29576847 Free PMC article.
References
-
- Guidarelli A., Sciorati C., Clementi E., Cantoni O. Peroxynitrite mobilizes calcium ions from ryanodine-sensitive stores, a process associated with the mitochondrial accumulation of the cation and the enforced formation of species mediating cleavage of genomic DNA. Free Radic. Biol. Med. 2006;41:154–164. doi: 10.1016/j.freeradbiomed.2006.03.023. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

