Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase
- PMID: 28767072
- PMCID: PMC5577573
- DOI: 10.3390/toxins9080239
Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase
Abstract
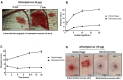
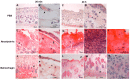
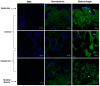
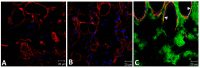
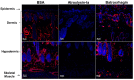
Hemorrhage is the most prominent effect of snake venom metalloproteinases (SVMPs) in human envenomation. The capillary injury is a multifactorial effect caused by hydrolysis of the components of the basement membrane (BM). The PI and PIII classes of SVMPs are abundant in viperid venoms and hydrolyze BM components. However, hemorrhage is associated mostly with PIII-class SVMPs that contain non-catalytic domains responsible for the binding of SVMPs to BM proteins, facilitating enzyme accumulation in the tissue and enhancing its catalytic efficiency. Here we report on Atroxlysin-Ia, a PI-class SVMP that induces hemorrhagic lesions in levels comparable to those induced by Batroxrhagin (PIII-class), and a unique SVMP effect characterized by the rapid onset of dermonecrotic lesions. Atroxlysin-Ia was purified from B. atrox venom, and sequence analyses indicated that it is devoid of non-catalytic domains and unable to bind to BM proteins as collagen IV and laminin in vitro or in vivo. The presence of Atroxlysin-Ia was diffuse in mice skin, and localized mainly in the epidermis with no co-localization with BM components. Nevertheless, the skin lesions induced by Atroxlysin-Ia were comparable to those induced by Batroxrhagin, with induction of leukocyte infiltrates and hemorrhagic areas soon after toxin injection. Detachment of the epidermis was more intense in skin injected with Atroxlysin-Ia. Comparing the catalytic activity of both toxins, Batroxrhagin was more active in the hydrolysis of a peptide substrate while Atroxlysin-Ia hydrolyzed more efficiently fibrin, laminin, collagen IV and nidogen. Thus, the results suggest that Atroxlysin-Ia bypasses the binding step to BM proteins, essential for hemorrhagic lesions induced by PII- and P-III class SVMPs, causing a significantly fast onset of hemorrhage and dermonecrosis, due to its higher proteolytic capacity on BM components.
Keywords: Bothrops atrox; dermonecrosis; extracellular matrix; hemorrhage; metalloproteinase; snake; venom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



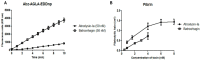


Similar articles
-
Role of collagens and perlecan in microvascular stability: exploring the mechanism of capillary vessel damage by snake venom metalloproteinases.PLoS One. 2011;6(12):e28017. doi: 10.1371/journal.pone.0028017. Epub 2011 Dec 8. PLoS One. 2011. PMID: 22174764 Free PMC article.
-
Inflammatory Reaction Induced by Two Metalloproteinases Isolated from Bothrops atrox Venom and by Fragments Generated from the Hydrolysis of Basement Membrane Components.Toxins (Basel). 2020 Feb 2;12(2):96. doi: 10.3390/toxins12020096. Toxins (Basel). 2020. PMID: 32024243 Free PMC article.
-
The novel metalloproteinase atroxlysin-I from Peruvian Bothrops atrox (Jergón) snake venom acts both on blood vessel ECM and platelets.Arch Biochem Biophys. 2010 Apr 1;496(1):9-20. doi: 10.1016/j.abb.2010.01.010. Epub 2010 Jan 25. Arch Biochem Biophys. 2010. PMID: 20102699
-
Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases.J Proteomics. 2011 Aug 24;74(9):1781-94. doi: 10.1016/j.jprot.2011.03.026. Epub 2011 Apr 6. J Proteomics. 2011. PMID: 21447411 Review.
-
Hemorrhage induced by snake venom metalloproteinases: biochemical and biophysical mechanisms involved in microvessel damage.Toxicon. 2005 Jun 15;45(8):997-1011. doi: 10.1016/j.toxicon.2005.02.029. Epub 2005 Apr 18. Toxicon. 2005. PMID: 15922771 Review.
Cited by
-
Accuracy of the Lee-White Clotting Time Performed in the Hospital Routine to Detect Coagulopathy in Bothrops atrox Envenomation.Am J Trop Med Hyg. 2018 May;98(5):1547-1551. doi: 10.4269/ajtmh.17-0992. Epub 2018 Mar 29. Am J Trop Med Hyg. 2018. PMID: 29611503 Free PMC article.
-
Individual Variability in Bothropsatrox Snakes Collected from Different Habitats in the Brazilian Amazon: New Findings on Venom Composition and Functionality.Toxins (Basel). 2021 Nov 18;13(11):814. doi: 10.3390/toxins13110814. Toxins (Basel). 2021. PMID: 34822598 Free PMC article.
-
A Complex Pattern of Gene Expression in Tissue Affected by Viperid Snake Envenoming: The Emerging Role of Autophagy-Related Genes.Biomolecules. 2024 Feb 26;14(3):278. doi: 10.3390/biom14030278. Biomolecules. 2024. PMID: 38540699 Free PMC article.
-
Snake Venom Metalloproteinases from Puff Adder and Saw-Scaled Viper Venoms Cause Cytotoxic Effects in Human Keratinocytes.Toxins (Basel). 2025 Jun 28;17(7):328. doi: 10.3390/toxins17070328. Toxins (Basel). 2025. PMID: 40711139 Free PMC article.
-
Antivenom Production against Bothrops jararaca and Bothrops erythromelas Snake Venoms Using Cross-Linked Chitosan Nanoparticles as an Immunoadjuvant.Toxins (Basel). 2018 Apr 16;10(4):158. doi: 10.3390/toxins10040158. Toxins (Basel). 2018. PMID: 29659491 Free PMC article.
References
-
- Gutiérrez J.M., Romero M., Núñez J., Chaves F., Borkow G., Ovadia M. Skeletal muscle necrosis and regeneration after injection of bah1, a hemorrhagic metalloproteinase isolated from the venom of the snake Bothrops asper (terciopelo) Exp. Mol. Pathol. 1995;62:28–41. doi: 10.1006/exmp.1995.1004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

