Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes
- PMID: 28767098
- PMCID: PMC5578073
- DOI: 10.3390/ijms18081683
Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes
Abstract
Nucleic acid aptamers have minimal immunogenicity, high chemical synthesis production, low cost and high chemical stability when compared with antibodies. However, the susceptibility to nuclease degradation, rapid excretion through renal filtration and insufficient binding affinity hindered their development as drug candidates for therapeutic applications. In this review, we will discuss methods to conquer these challenges and highlight recent developments of chemical modifications and technological advances that may enable early aptamers to be translated into clinical therapeutics.
Keywords: binding affinity; chemical modification; nuclease degradation; nucleic acid aptamer; rapid excretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








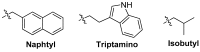

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

