Total Synthesis of the Schisandraceae Nortriterpenoid Rubriflordilactone A
- PMID: 28768051
- PMCID: PMC5656881
- DOI: 10.1002/chem.201703229
Total Synthesis of the Schisandraceae Nortriterpenoid Rubriflordilactone A
Abstract
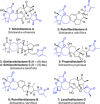
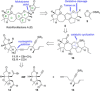
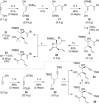
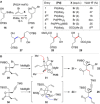
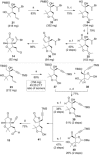
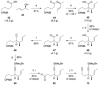
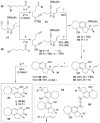
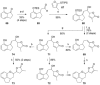
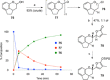
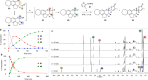
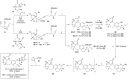
Full details of the total synthesis of the Schisandraceae nortriterpenoid natural product rubriflordilactone A are reported. Palladium- and cobalt-catalyzed polycyclizations were employed as key strategies to construct the central pentasubstituted arene from bromoendiyne and triyne precursors. This required the independent assembly of two AB ring aldehydes for combination with a common diyne component. A number of model systems were explored to investigate these two methodologies, and also to establish routes for the installation of the challenging benzopyran and butenolide rings.
Keywords: cascade cyclization; cyclotrimerization; natural products; total synthesis; transition-metal catalysis.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
Similar articles
-
Total Synthesis of (+)-Rubriflordilactone A.Angew Chem Int Ed Engl. 2015 Oct 19;54(43):12618-21. doi: 10.1002/anie.201506366. Epub 2015 Sep 4. Angew Chem Int Ed Engl. 2015. PMID: 26337920 Free PMC article.
-
Diastereoselective total synthesis of (±)-schindilactone A.Angew Chem Int Ed Engl. 2011 Aug 1;50(32):7373-7. doi: 10.1002/anie.201103088. Epub 2011 Jul 7. Angew Chem Int Ed Engl. 2011. PMID: 21739547 No abstract available.
-
Metal-catalyzed syntheses of abridged CDE rings of rubriflordilactones A and B.Org Lett. 2012 Dec 21;14(24):6278-81. doi: 10.1021/ol303041j. Epub 2012 Dec 4. Org Lett. 2012. PMID: 23210932
-
Triterpenoids from the Schisandraceae family.Nat Prod Rep. 2008 Oct;25(5):871-91. doi: 10.1039/b719905h. Epub 2008 Jul 31. Nat Prod Rep. 2008. PMID: 18820756 Review.
-
Triterpenoids from the Schisandraceae family: an update.Nat Prod Rep. 2015 Mar;32(3):367-410. doi: 10.1039/c4np00117f. Nat Prod Rep. 2015. PMID: 25483912 Review.
Cited by
-
New Developments of the Principle of Vinylogy as Applied to π-Extended Enolate-Type Donor Systems.Chem Rev. 2020 Mar 11;120(5):2448-2612. doi: 10.1021/acs.chemrev.9b00481. Epub 2020 Feb 10. Chem Rev. 2020. PMID: 32040305 Free PMC article.
-
Total Synthesis of Natural Terpenoids Enabled by Cobalt Catalysis.Front Chem. 2022 Jun 15;10:941184. doi: 10.3389/fchem.2022.941184. eCollection 2022. Front Chem. 2022. PMID: 35783212 Free PMC article. Review.
-
Convergent Total Syntheses of (-)-Rubriflordilactone B and (-)-pseudo-Rubriflordilactone B.Angew Chem Int Ed Engl. 2019 Dec 9;58(50):18177-18181. doi: 10.1002/anie.201908917. Epub 2019 Oct 31. Angew Chem Int Ed Engl. 2019. PMID: 31595605 Free PMC article.
References
-
- Hancke J. L., Burgos R. A., Ahumada F., Fitoterapia 1999, 70, 451.
-
- None
-
- For reviews on Schisandraceae natural products, see: Li X., Cheong P. H.-Y., Carter R. G., Angew. Chem. Int. Ed. 2017, 56, 1704;
- Angew. Chem. 2017, 129, 1728;
-
- Shi Y.-M., Xiao W.-L., Pu J.-X., Sun H.-D., Nat. Prod. Rep. 2015, 32, 367; - PubMed
-
- For reviews, see: Xia Y.-G., Yang B.-Y., Kuang H.-X., Phytochem. Rev. 2015, 14, 155;
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

