A Multidimensional B-Spline Correction for Accurate Modeling Sugar Puckering in QM/MM Simulations
- PMID: 28768099
- PMCID: PMC5839098
- DOI: 10.1021/acs.jctc.7b00161
A Multidimensional B-Spline Correction for Accurate Modeling Sugar Puckering in QM/MM Simulations
Abstract
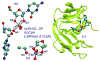
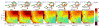
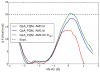
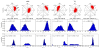
The computational efficiency of approximate quantum mechanical methods allows their use for the construction of multidimensional reaction free energy profiles. It has recently been demonstrated that quantum models based on the neglect of diatomic differential overlap (NNDO) approximation have difficulty modeling deoxyribose and ribose sugar ring puckers and thus limit their predictive value in the study of RNA and DNA systems. A method has been introduced in our previous work to improve the description of the sugar puckering conformational landscape that uses a multidimensional B-spline correction map (BMAP correction) for systems involving intrinsically coupled torsion angles. This method greatly improved the adiabatic potential energy surface profiles of DNA and RNA sugar rings relative to high-level ab initio methods even for highly problematic NDDO-based models. In the present work, a BMAP correction is developed, implemented, and tested in molecular dynamics simulations using the AM1/d-PhoT semiempirical Hamiltonian for biological phosphoryl transfer reactions. Results are presented for gas-phase adiabatic potential energy surfaces of RNA transesterification model reactions and condensed-phase QM/MM free energy surfaces for nonenzymatic and RNase A-catalyzed transesterification reactions. The results show that the BMAP correction is stable, efficient, and leads to improvement in both the potential energy and free energy profiles for the reactions studied, as compared with ab initio and experimental reference data. Exploration of the effect of the size of the quantum mechanical region indicates the best agreement with experimental reaction barriers occurs when the full CpA dinucleotide substrate is treated quantum mechanically with the sugar pucker correction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2. Chapter 1. Cold Spring Harbor; New York: 2009. pp. 1–22. - PubMed
-
- Bloomfield VA, Crothers DM, Tinoco I., Jr . Nucleic Acids: Structures, Properties, and Functions. Chapters 2 and 5. University Science Books; Sausalito, CA: 2000. pp. 13–44.pp. 111–164.
-
- Saenger W. Principles of Nucleic Acid Structure. Chapters 2 and 17. Springer-Verlag; New York: 1984. pp. 17–21.pp. 368–384.
-
- Rich A. The double helix: a tale of two puckers. Nat Struct Biol. 2003;10:247–249. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
