The effect of glycopyrronium and indacaterol, as monotherapy and in combination, on the methacholine dose-response curve of mild asthmatics: a randomized three-way crossover study
- PMID: 28768531
- PMCID: PMC5541419
- DOI: 10.1186/s12931-017-0628-4
The effect of glycopyrronium and indacaterol, as monotherapy and in combination, on the methacholine dose-response curve of mild asthmatics: a randomized three-way crossover study
Abstract
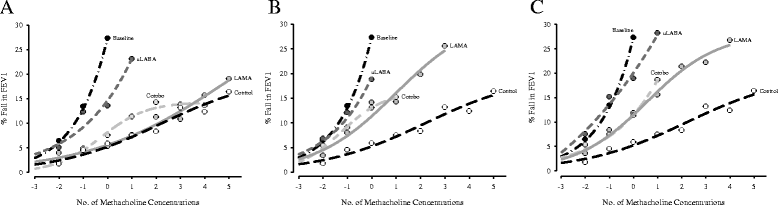
Background: Methacholine dose-response curves illustrate pharmacologic bronchoprotection against methacholine-induced airway hyperresponsiveness and can be used to quantitate changes in airway sensitivity (position), reactivity (slope), and maximal responsiveness following drug administration. Our objective was to determine the influence of single-dose glycopyrronium (long-acting muscarinic antagonist) and indacaterol (ultra-long acting β2 agonist), as monotherapy and in combination, on the methacholine dose-response curve of mild asthmatics and to compare these findings with a non-asthmatic control curve.
Methods: This was a randomized, double blind, double dummy, three-way crossover study. For asthmatic participants (n = 14), each treatment arm included a baseline methacholine challenge, drug administration, and repeat methacholine challenges at 1, 24, and 48 h. Non-asthmatic control participants (n = 15) underwent a single methacholine challenge and did not receive any study treatment. Methacholine dose-response curves were graphed as the percent fall in forced expiratory volume in 1 s (FEV1) for each methacholine concentration administered. Best-fit curves were then generated. Differences in airway reactivity were calculated through linear regression. Changes in airway sensitivity were assessed as the shift in the provocative concentration of methacholine causing a 20% fall in FEV1.
Results: Compared to baseline, all treatments significantly reduced airway sensitivity to methacholine at 1 h post-dose (indacaterol ~1.5 doubling concentrations; glycopyrronium ~5 doubling concentrations; combination ~5 doubling concentrations). Bronchoprotection at 24 and 48 h remained significant with glycopyrronium and combination therapy only. Airway reactivity was not influenced by indacaterol whereas glycopyrronium significantly reduced airway reactivity at all time-points (p = 0.003-0.027). The combination significantly decreased slope at 1 (p = 0.021) and 24 (p = 0.039) hours only. The non-asthmatic control and 1-h glycopyrronium curves are nearly identical. Only the non-asthmatic control and 1-h post-combination therapy curves appeared to generate a true response plateau (three data points within 5%), which occurred at a 14% fall in FEV1.
Conclusions: Methacholine dose-response curves differentiate the bronchoprotective mechanisms triggered by different classes of asthma medications. Assessment of bronchoprotection using methacholine dose-response curves may be useful during clinical development of respiratory medications when performing superiority, equivalence, or non-inferiority trials.
Trial registration: clinicaltrials.gov ( NCT02953041 ). Retrospectively registered on October 24th 2016.
Keywords: Combination therapy; Long-acting muscarinic antagonist; Ultra-long acting β2 agonist.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the University of Saskatchewan Biomedical Research Ethics Board (Bio REB 16-205) and was registered with
Consent for publication
All participants consented to have their individual data used and disclosed for publication purposes as long as the information was de-identified.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Sterk PJ, Bel EH. Bronchial hyperresponsiveness: the need for a distinction between hypersensitivity and excessive airway narrowing. Eur Respir J. 1989;2:267–274. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

