TREM2 in Neurodegenerative Diseases
- PMID: 28768545
- PMCID: PMC5541421
- DOI: 10.1186/s13024-017-0197-5
TREM2 in Neurodegenerative Diseases
Abstract
TREM2 variants have been identified as risk factors for Alzheimer's disease (AD) and other neurodegenerative diseases (NDDs). Because TREM2 encodes a receptor exclusively expressed on immune cells, identification of these variants conclusively demonstrates that the immune response can play an active role in the pathogenesis of NDDs. These TREM2 variants also confer the highest risk for developing Alzheimer's disease of any risk factor identified in nearly two decades, suggesting that understanding more about TREM2 function could provide key insights into NDD pathology and provide avenues for novel immune-related NDD biomarkers and therapeutics. The expression, signaling and function of TREM2 in NDDs have been extensively investigated in an effort to understand the role of immune function in disease pathogenesis and progression. We provide a comprehensive review of our current understanding of TREM2 biology, including new insights into the regulation of TREM2 expression, and TREM2 signaling and function across NDDs. While many open questions remain, the current body of literature provides clarity on several issues. While it is still often cited that TREM2 expression is decreased by pro-inflammatory stimuli, it is now clear that this is true in vitro, but inflammatory stimuli in vivo almost universally increase TREM2 expression. Likewise, while TREM2 function is classically described as promoting an anti-inflammatory phenotype, more than half of published studies demonstrate a pro-inflammatory role for TREM2, suggesting that its role in inflammation is much more complex. Finally, these components of TREM2 biology are applied to a discussion of how TREM2 impacts NDD pathologies and the latest assessment of how these findings might be applied to immune-directed clinical biomarkers and therapeutics.
Keywords: Alzheimer’s disease; Frontotemporal dementia; Genetic risk factors; Genetics; Inflammation; Microglia; Neurodegeneration; Parkinson’s disease; Triggering receptor expressed on myeloid cells 2.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
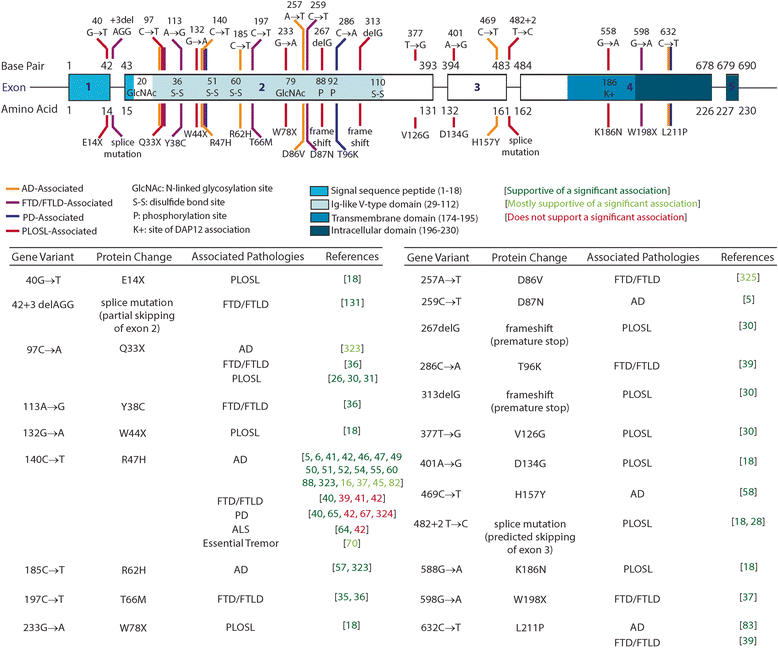
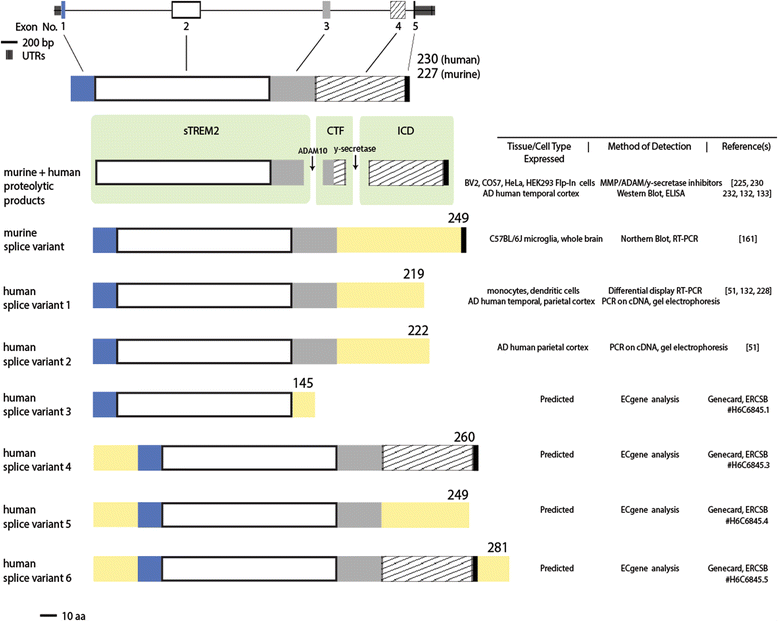
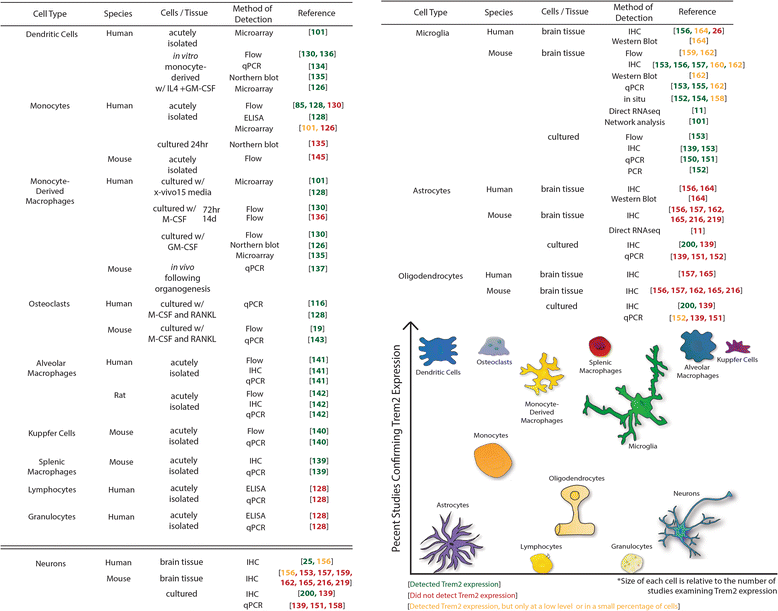
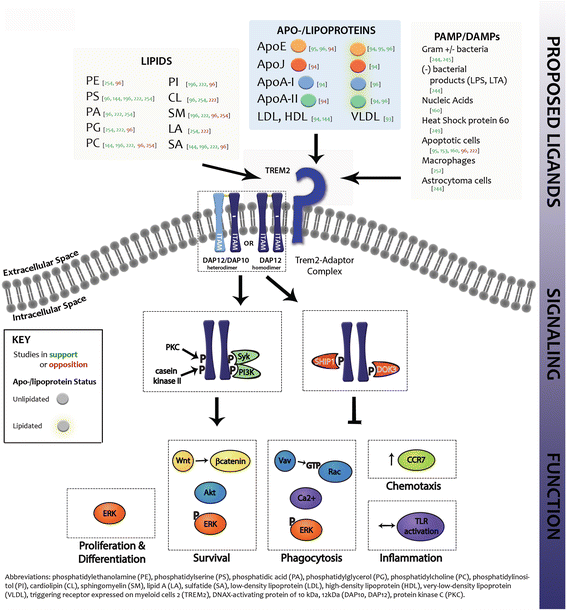
References
-
- Grupe A, Abraham R, Li Y, Rowland C, Hollingworth P, Morgan A, Jehu L, Segurado R, Stone D, Schadt E, et al. Evidence for novel susceptibility genes for late-onset Alzheimer’s disease from a genome-wide association study of putative functional variants. Hum Mol Genet. 2007;16:865–873. doi: 10.1093/hmg/ddm031. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

