Pathophysiology of thrombotic thrombocytopenic purpura
- PMID: 28768626
- PMCID: PMC5606001
- DOI: 10.1182/blood-2017-04-636431
Pathophysiology of thrombotic thrombocytopenic purpura
Abstract
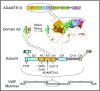
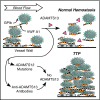
The discovery of a disintegrin-like and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13) revolutionized our approach to thrombotic thrombocytopenic purpura (TTP). Inherited or acquired ADAMTS13 deficiency allows the unrestrained growth of microthrombi that are composed of von Willebrand factor and platelets, which account for the thrombocytopenia, hemolytic anemia, schistocytes, and tissue injury that characterize TTP. Most patients with acquired TTP respond to a combination of plasma exchange and rituximab, but some die or acquire irreversible neurological deficits before they can respond, and relapses can occur unpredictably. However, knowledge of the pathophysiology of TTP has inspired new ways to prevent early deaths by targeting autoantibody production, replenishing ADAMTS13, and blocking microvascular thrombosis despite persistent ADAMTS13 deficiency. In addition, monitoring ADAMTS13 has the potential to identify patients who are at risk of relapse in time for preventive therapy.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.E.S. is a consultant for Ablynx, BioMarin, 23andMe, and Genentech.
Figures

References
-
- Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45(2):139-159.
-
- Rock GA, Shumak KH, Buskard NA, et al. ; Canadian Apheresis Study Group. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325(6):393-397. - PubMed
-
- Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307(23):1432-1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

