A solute-binding protein in the closed conformation induces ATP hydrolysis in a bacterial ATP-binding cassette transporter involved in the import of alginate
- PMID: 28768763
- PMCID: PMC5612102
- DOI: 10.1074/jbc.M117.793992
A solute-binding protein in the closed conformation induces ATP hydrolysis in a bacterial ATP-binding cassette transporter involved in the import of alginate
Abstract
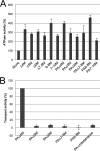
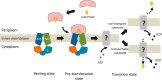
The Gram-negative bacterium Sphingomonas sp. A1 incorporates alginate into cells via the cell-surface pit without prior depolymerization by extracellular enzymes. Alginate import across cytoplasmic membranes thereby depends on the ATP-binding cassette transporter AlgM1M2SS (a heterotetramer of AlgM1, AlgM2, and AlgS), which cooperates with the periplasmic solute-binding protein AlgQ1 or AlgQ2; however, several details of AlgM1M2SS-mediated alginate import are not well-understood. Herein, we analyzed ATPase and transport activities of AlgM1M2SS after reconstitution into liposomes with AlgQ2 and alginate oligosaccharide substrates having different polymerization degrees (PDs). Longer alginate oligosaccharides (PD ≥ 5) stimulated the ATPase activity of AlgM1M2SS but were inert as substrates of AlgM1M2SS-mediated transport, indicating that AlgM1M2SS-mediated ATP hydrolysis can be stimulated independently of substrate transport. Using X-ray crystallography in the presence of AlgQ2 and long alginate oligosaccharides (PD 6-8) and with the humid air and glue-coating method, we determined the crystal structure of AlgM1M2SS in complex with oligosaccharide-bound AlgQ2 at 3.6 Å resolution. The structure of the ATP-binding cassette transporter in complex with non-transport ligand-bound periplasmic solute-binding protein revealed that AlgM1M2SS and AlgQ2 adopt inward-facing and closed conformations, respectively. These in vitro assays and structural analyses indicated that interactions between AlgM1M2SS in the inward-facing conformation and periplasmic ligand-bound AlgQ2 in the closed conformation induce ATP hydrolysis by the ATP-binding protein AlgS. We conclude that substrate-bound AlgQ2 in the closed conformation initially interacts with AlgM1M2SS, the AlgM1M2SS-AlgQ2 complex then forms, and this formation is followed by ATP hydrolysis.
Keywords: ABC transporter; ATPase; X-ray crystallography; bacteria; crystal structure.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


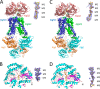

References
-
- Beis K. (2015) Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 43, 889–893 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

