Factors Associated With Time to Site Activation, Randomization, and Enrollment Performance in a Stroke Prevention Trial
- PMID: 28768800
- PMCID: PMC5575963
- DOI: 10.1161/STROKEAHA.117.016976
Factors Associated With Time to Site Activation, Randomization, and Enrollment Performance in a Stroke Prevention Trial
Abstract
Background and purpose: Multicenter clinical trials attempt to select sites that can move rapidly to randomization and enroll sufficient numbers of patients. However, there are few assessments of the success of site selection.
Methods: In the CREST-2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trials), we assess factors associated with the time between site selection and authorization to randomize, the time between authorization to randomize and the first randomization, and the average number of randomizations per site per month. Potential factors included characteristics of the site, specialty of the principal investigator, and site type.
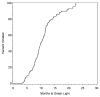
Results: For 147 sites, the median time between site selection to authorization to randomize was 9.9 months (interquartile range, 7.7, 12.4), and factors associated with early site activation were not identified. The median time between authorization to randomize and a randomization was 4.6 months (interquartile range, 2.6, 10.5). Sites with authorization to randomize in only the carotid endarterectomy study were slower to randomize, and other factors examined were not significantly associated with time-to-randomization. The recruitment rate was 0.26 (95% confidence interval, 0.23-0.28) patients per site per month. By univariate analysis, factors associated with faster recruitment were authorization to randomize in both trials, principal investigator specialties of interventional radiology and cardiology, pre-trial reported performance >50 carotid angioplasty and stenting procedures per year, status in the top half of recruitment in the CREST trial, and classification as a private health facility. Participation in StrokeNet was associated with slower recruitment as compared with the non-StrokeNet sites.
Conclusions: Overall, selection of sites with high enrollment rates will likely require customization to align the sites selected to the factor under study in the trial.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02089217.
Keywords: carotid arteries; carotid stenosis; clinical trial; endarterectomy, carotid; multicenter study; randomized controlled trial; vascular diseases.
© 2017 American Heart Association, Inc.
Figures



Comment in
-
Unlocking the Keys to Site Activation and Recruitment Success in a Randomized Controlled Trial.Stroke. 2017 Sep;48(9):2339-2340. doi: 10.1161/STROKEAHA.117.018306. Epub 2017 Aug 2. Stroke. 2017. PMID: 28768798 Free PMC article. No abstract available.
Similar articles
-
Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials.Int J Stroke. 2017 Oct;12(7):770-778. doi: 10.1177/1747493017706238. Epub 2017 May 2. Int J Stroke. 2017. PMID: 28462683 Free PMC article. Clinical Trial.
-
Clinical need, design, and goals for the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis trial.Semin Vasc Surg. 2017 Mar;30(1):2-7. doi: 10.1053/j.semvascsurg.2017.04.004. Epub 2017 Apr 27. Semin Vasc Surg. 2017. PMID: 28818255 Clinical Trial.
-
Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis.N Engl J Med. 2016 Mar 17;374(11):1011-20. doi: 10.1056/NEJMoa1515706. Epub 2016 Feb 17. N Engl J Med. 2016. PMID: 26886419 Clinical Trial.
-
Endarterectomy or carotid artery stenting: the quest continues.Am J Surg. 2008 Feb;195(2):259-69. doi: 10.1016/j.amjsurg.2007.07.022. Am J Surg. 2008. PMID: 18154764 Review.
-
Issues to Be Addressed and Hopefully Resolved in the Carotid Revascularization Endarterectomy Versus Stenting Trial 2.Angiology. 2016 May;67(5):408-10. doi: 10.1177/0003319715611281. Epub 2015 Oct 11. Angiology. 2016. PMID: 26460327 Review.
Cited by
-
Enhancing site selection strategies in clinical trial recruitment using real-world data modeling.PLoS One. 2024 Mar 11;19(3):e0300109. doi: 10.1371/journal.pone.0300109. eCollection 2024. PLoS One. 2024. PMID: 38466688 Free PMC article.
-
Increasing Efficiency of Recruitment in Early Parkinson's Disease Trials: A Case Study Examination of the STEADY-PD III Trial.J Parkinsons Dis. 2017;7(4):685-693. doi: 10.3233/JPD-171199. J Parkinsons Dis. 2017. PMID: 29103052 Free PMC article. Clinical Trial.
-
Unlocking the Keys to Site Activation and Recruitment Success in a Randomized Controlled Trial.Stroke. 2017 Sep;48(9):2339-2340. doi: 10.1161/STROKEAHA.117.018306. Epub 2017 Aug 2. Stroke. 2017. PMID: 28768798 Free PMC article. No abstract available.
-
Selecting trial centers using a standardized, automated site assessment survey instrument (SASI).Contemp Clin Trials. 2024 Aug;143:107583. doi: 10.1016/j.cct.2024.107583. Epub 2024 May 29. Contemp Clin Trials. 2024. PMID: 38821259 Free PMC article.
References
-
- Harper BD, Zuckerman D SHEP Cooperative Research Group. Critical success factor for planning for site selection and patient recruitment planning. BioExecutive International. 2006;2:S16–S28.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

