Dichotomous miR expression and immune responses following primary blood-stage malaria
- PMID: 28768914
- PMCID: PMC5543925
- DOI: 10.1172/jci.insight.93434
Dichotomous miR expression and immune responses following primary blood-stage malaria
Abstract
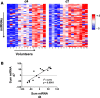
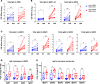
Clinical responses to infection or vaccination and the development of effective immunity are characterized in humans by a marked interindividual variability. To gain an insight into the factors affecting this variability, we used a controlled human infection system to study early immune events following primary infection of healthy human volunteers with blood-stage Plasmodium falciparum malaria. By day 4 of infection, a dichotomous pattern of high or low expression of a defined set of microRNAs (miRs) emerged in volunteers that correlated with variation in parasite growth rate. Moreover, high-miR responders had higher numbers of activated CD4+ T cells, and developed significantly enhanced antimalarial antibody responses. Notably, a set of 17 miRs was identified in the whole blood of low-miR responders prior to infection that differentiated them from high-miR responders. These data implicate preexisting host factors as major determinants in the ability to effectively respond to primary malaria infection.
Keywords: Immunology; Infectious disease.
Conflict of interest statement
Figures




References
-
- WHO. World Malaria Report 2015. Geneva, Switzerland: WHO; 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report... Accessed July 13, 2017.
-
- Dye C, Floyd K. Tuberculosis. In: Jamison DT, et al., eds. Disease Control Priorities in Developing Countries. Washington, DC, USA: World Bank Publications; 2006:Chapter 16.
-
- Garnham PCC. Charles Nicolle and inapparent infections. Am J Trop Med Hyg. 1977;26:1101–1104. doi: 10.4269/ajtmh.1977.26.1101. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

