Pediatric Craniovertebral Junction Surgery
- PMID: 28768919
- PMCID: PMC5638788
- DOI: 10.2176/nmc.ra.2017-0032
Pediatric Craniovertebral Junction Surgery
Abstract
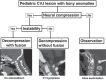
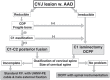
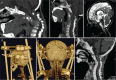
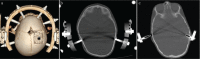
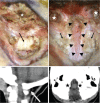
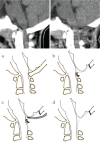
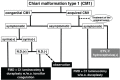
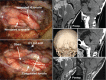
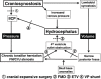
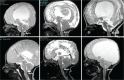
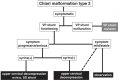
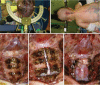
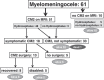
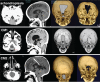
The craniovertebral junction (CVJ) has attracted more attention in pediatric medicine in recent years due to the progress in surgical technologies allowing a direct approach to the CVJ in children. The CVJ is the site of numerous pathologies, most originating in bone anomalies resulting from abnormal CVJ development. Before discussing the surgical approaches to CVJ, three points should be borne in mind: first, that developmental anatomy demonstrates age-dependent mechanisms and the pathophysiology of pediatric CVJ anomalies; second, that CT-based dynamic simulations have improved our knowledge of functional anatomy, enabling us to locate CVJ lesions with greater certainty; and third, understanding the complex structure of the pediatric CVJ also clarifies the surgical anatomy. This review begins with a description of the embryonic developmental process of the CVJ, comprising ossification and resegmentation of the somite. From the clinical perspective, pediatric CVJ lesions can be divided into three categories: developmental bony anomalies with or without instability, stenotic CVJ lesions, and others. After discussing surgery and management based on this classification, the author describes surgical outcomes on his hands, and finally proceeds to address controversial issues specific for pediatric CVJ surgery. The lessons, which the author has gleaned from his experience in pediatric CVJ surgery, are also presented briefly in this review. Recent technological progress has facilitated pediatric surgery of the CVJ. However, it is important to recognize that we are still far from reliably and consistently obtaining satisfactory results. Further progress in this area awaits contributions of the coming generations of pediatric surgeons.
Keywords: congenital anomaly; craniovertebral junction; embryology; pediatrics; surgery.
Conflict of interest statement
The author has no conflicts of interest regarding this manuscript and has registered online Self-reported COI Disclosure Statement Forms through the website for the Japan Neurosurgical Society (JNS) members.
Figures






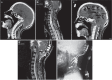
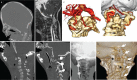
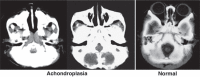
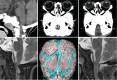

References
-
- Morota N: Intraoperative neurophysiological monitoring in surgery for the craniovertebral junction. In Goel A, Cacciola F. (eds): The craniovertebral junction. Stuttgart, Thieme, 2011, pp. 194–205
-
- Cacciola F, Di Lorenzo N: Embryology and development of the craniovertebral junction. In Goel A, Cacciola F. (eds): The craniovertebral junction. Stuttgart, Thieme, 2011, pp. 14–20
-
- Menezes AH: Craniocervical developmental anatomy and its implications. Childs Nerv Syst 24: 1109–1122, 2008 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

