Characterisation of lung macrophage subpopulations in COPD patients and controls
- PMID: 28769058
- PMCID: PMC5540919
- DOI: 10.1038/s41598-017-07101-2
Characterisation of lung macrophage subpopulations in COPD patients and controls
Abstract
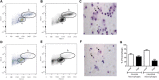
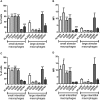
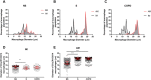
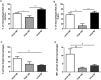
Lung macrophage subpopulations have been identified based on size. We investigated characteristics of small and large macrophages in the alveolar spaces and lung interstitium of COPD patients and controls. Alveolar and interstitial cells were isolated from lung resection tissue from 88 patients. Macrophage subpopulation cell-surface expression of immunological markers and phagocytic ability were assessed by flow cytometry. Inflammatory related gene expression was measured. Alveolar and interstitial macrophages had subpopulations of small and large macrophages based on size and granularity. Alveolar macrophages had similar numbers of small and large cells; interstitial macrophages were mainly small. Small macrophages expressed significantly higher cell surface HLA-DR, CD14, CD38 and CD36 and lower CD206 compared to large macrophages. Large alveolar macrophages showed lower marker expression in COPD current compared to ex-smokers. Small interstitial macrophages had the highest pro-inflammatory gene expression levels, while large alveolar macrophages had the lowest. Small alveolar macrophages had the highest phagocytic ability. Small alveolar macrophage CD206 expression was lower in COPD patients compared to smokers. COPD lung macrophages include distinct subpopulations; Small interstitial and small alveolar macrophages with more pro-inflammatory and phagocytic function respectively, and large alveolar macrophages with low pro-inflammatory and phagocytic ability.
Conflict of interest statement
D. Singh has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Skypharma, Takeda, Teva, Therevance and Verona.E. Hardaker was employed by Novartis during the undertaking of this project. S. Lea, J. Dewhurst, J. Dungwa and A. Ravi declare that they have no competing interests
Figures


References
-
- Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

