Metformin Inhibits Cyst Formation in a Zebrafish Model of Polycystin-2 Deficiency
- PMID: 28769124
- PMCID: PMC5541071
- DOI: 10.1038/s41598-017-07300-x
Metformin Inhibits Cyst Formation in a Zebrafish Model of Polycystin-2 Deficiency
Abstract
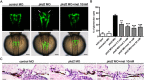
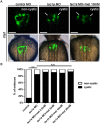
Autosomal dominant polycystic kidney disease (ADPKD) is a common kidney disease caused by mutations in PKD1 or PKD2. Metformin reduces cyst growth in mouse models of PKD1. However, metformin has not been studied in animal models of PKD2, and the cellular mechanism underlying its effectiveness is not entirely clear. This study investigated the effects of metformin on cyst formation in a zebrafish model of polycystin-2 deficiency resulting from morpholino knockdown of pkd2. We added metformin (2.5 to 20 mM) to the embryo media between 4 and 48 hours post fertilisation and observed pronephric cyst formation by using the wt1b promoter-driven GFP signal in Tg(wt1b:GFP) pkd2 morphants. Metformin inhibited pronephric cyst formation by 42-61% compared with the untreated controls. Metformin also reduced the number of proliferating cells in the pronephric ducts, the degree of dorsal body curvature, and the infiltration of leukocytes surrounding the pronephros. Moreover, metformin treatment increased the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and enhanced autophagy in the pronephros. Our data suggest that metformin reduces cyst formation through activation of the AMPK pathway and modulation of defective cellular events such as proliferation and autophagy. These results also imply that metformin could have therapeutic potential for ADPKD treatment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

