Intravitreal aflibercept for choroidal neovascularization associated with chorioretinitis: a pilot study
- PMID: 28769551
- PMCID: PMC5533470
- DOI: 10.2147/OPTH.S132923
Intravitreal aflibercept for choroidal neovascularization associated with chorioretinitis: a pilot study
Erratum in
-
Erratum: Intravitreal aflibercept for choroidal neovascularization associated with chorioretinitis: a pilot study [Corrigendum].Clin Ophthalmol. 2017 Aug 28;11:1567. doi: 10.2147/OPTH.S148326. eCollection 2017. Clin Ophthalmol. 2017. PMID: 28894355 Free PMC article.
Abstract
Purpose: The purpose of this study was to evaluate the potential benefits of intravitreal aflibercept injections for the treatment of choroidal neovascularization (CNV) secondary to chorioretinitis.
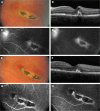
Methods: In this uncontrolled, prospective cohort study, 15 eyes of 14 consecutive patients affected by CNV associated with ocular toxoplasmosis were treated with intravitreal aflibercept (2 mg) pro re nata and observed over a 12-month follow-up period. The primary outcome was the change in best-corrected visual acuity (BCVA) from baseline to month 12. Secondary outcomes included change in central retinal thickness (CRT) in the foveal area on optical coherence tomography (OCT) from baseline to month 12, the number of intravitreal aflibercept injections administered, and safety.
Results: Mean (standard deviation [SD]) BCVA improved significantly from 0.36 (0.23) at baseline to 0.64 (0.3) at month 12 (P=0.0002). Mean (SD) CRT on OCT showed a reduction from 317 (74) µm at baseline to 254 (43) µm (P=0.0002) at month 12. A mean (SD) of 1.7 (0.5) injections (range, 1-2 injections) were performed during the study period. No cases of endophthalmitis, uveitis, stroke, or retinal detachment were noted. No patient demonstrated an intraocular pressure >20 mmHg at any study visit.
Conclusion: Intravitreal aflibercept showed a positive clinical effect and was well tolerated for the treatment of CNV associated with chorioretinitis. The results could be helpful for selecting a treatment for CNV secondary to chorioretinitis.
Keywords: aflibercept; angiogenesis inhibitors; anti-VEGF; central chori-oretinitis; choroidal neovascularization; toxoplasmosis.
Conflict of interest statement
Disclosure All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript. The authors report no conflicts of interest in this work.
Figures
References
-
- Mauget-Faÿsse M, Mimoun G, Ruiz-Moreno JM, et al. Verteporfin photodynamic therapy for choroidal neovascularization associated with toxoplasmic retinochoroiditis. Retina. 2006;26(4):396–403. - PubMed
-
- Neri P, Mercanti L, Mariotti C, Salvolini S, Giovannini A. Long-term control of choroidal neovascularization in quiescent congenital toxoplasma retinochoroiditis with photodynamic therapy: 4-year results. Int Ophthalmol. 2010;30(1):51–56. - PubMed
-
- Oliveira LB, Reis PA. Photodynamic therapy-treated choroidal neovascular membrane secondary to toxoplasmic retinochoroiditis. Graefes Arch Clin Exp Ophthalmol. 2004;242(12):1028–1030. - PubMed
-
- Arevalo JF, Adan A, Berrocal MH, et al. Intravitreal bevacizumab for inflammatory choroidal neovascularization: results from the Pan-American Collaborative Retina Study Group at 24 months. Retina. 2011;31(2):353–363. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

