Duloxetine 60 mg for chronic low back pain: post hoc responder analysis of double-blind, placebo-controlled trials
- PMID: 28769588
- PMCID: PMC5533563
- DOI: 10.2147/JPR.S138297
Duloxetine 60 mg for chronic low back pain: post hoc responder analysis of double-blind, placebo-controlled trials
Abstract
Introduction: Duloxetine has demonstrated efficacy in chronic low back pain (CLBP). We examined the predictors of response to duloxetine for CLBP.
Patients and methods: This was a post hoc analysis of pooled data from 4 double-blind, ran-domized, placebo-controlled trials of duloxetine (60 mg/day for 12-14 weeks) in adult patients with CLBP. Primary outcome was proportion of patients with ≥30% reduction in Brief Pain Inventory (BPI) average pain ("pain reduction") at 12-14 weeks. The proportion of patients with ≥30% and ≥50% (secondary outcome) pain reduction in duloxetine and placebo groups was compared. Variables for responder analyses were early improvement (≥15% pain reduction at Week 2), sex, age, baseline BPI average pain score, duration of CLBP, and number of painful body sites according to the Michigan Body Map (≥2 vs 1 [isolated CLBP]; 1 trial); relative risk (RR) and 95% confidence interval (CI) were calculated.
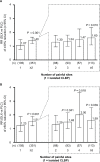
Results: Compared with placebo (n = 653), a greater proportion of duloxetine-treated patients (n = 642) achieved ≥30% (59.7% vs 47.8%; P < 0.001) and ≥50% pain reduction (48.6% vs 35.1%; P < 0.001). Among duloxetine-treated patients, early improvement was associated with greater likelihood of ≥30% (RR [95% CI], 2.91 [2.30-3.67]) or ≥50% (3.24 [2.44-4.31]) pain reduction. Women were slightly more likely than men to achieve ≥30% (RR [95% CI], 1.14 [1.00-1.30]) or ≥50% (1.17 [0.99-1.38]) pain reduction. Response rates were similar between age, CLBP duration, and baseline BPI average pain score subgroups. Patients with ≥2 painful sites were more likely to respond to duloxetine 60 mg relative to placebo than patients with isolated CLBP (RR, duloxetine vs placebo [95% CI]: ≥30% reduction, ≥2 painful sites 1.40 [1.18-1.66], isolated CLBP 1.07 [0.78-1.48]; ≥50% reduction, ≥2 painful sites 1.51 [1.20-1.89], isolated CLBP 1.23 [0.81-1.88]).
Conclusion: Early pain reduction was indicative of overall response. Patients with multiple painful sites had more benefit from duloxetine than patients with isolated CLBP.
Keywords: Brief Pain Inventory; Michigan Body Map; SNRI; chronic pain; low back pain; multiple painful sites.
Conflict of interest statement
Disclosure SK has received consulting fees and honoraria from Eli Lilly Japan K.K. and Shionogi & Co. Ltd and has received research funding from Shionogi & Co. Ltd. LA, HE, SF, KO, and AY are employees of Eli Lilly Japan K.K. MI and TT are employees and minor stock holders of Shionogi & Co. Ltd. LA and SF hold shares in Eli Lilly and Company. The authors report no other conflicts of interest in this work.
Figures


References
-
- Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. - PMC - PubMed
-
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. - PubMed
-
- Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

