Fluorescence Imaging of Streptococcus pneumoniae with the Helix pomatia agglutinin (HPA) As a Potential, Rapid Diagnostic Tool
- PMID: 28769901
- PMCID: PMC5513899
- DOI: 10.3389/fmicb.2017.01333
Fluorescence Imaging of Streptococcus pneumoniae with the Helix pomatia agglutinin (HPA) As a Potential, Rapid Diagnostic Tool
Abstract
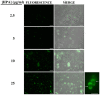
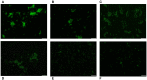
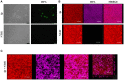
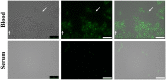
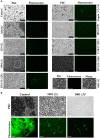
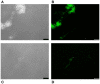
Streptococcus pneumoniae is a common human pathogen and a major causal agent of life-threatening infections that can either be respiratory or non-respiratory. It is well known that the Helix pomatia (edible snail) agglutinin (HPA) lectin shows specificity for terminal αGalNAc residues present, among other locations, in the Forssman pentasaccharide (αGalNAc1→3βGalNAc1→3αGal1→4βGal1→4βGlc). Based on experiments involving choline-independent mutants and different growth conditions, we propose here that HPA recognizes the αGalNAc terminal residues of the cell wall teichoic and lipoteichoic acids of S. pneumoniae. In addition, experimental evidence showing that pneumococci can be specifically labeled with HPA when growing as planktonic cultures as well as in mixed biofilms of S. pneumoniae and Haemophilus influenzae has been obtained. It should be underlined that pneumococci were HPA-labeled despite of the presence of a capsule. Although some non-pneumococcal species also bind the agglutinin, HPA-binding combined with fluorescence microscopy constitutes a suitable tool for identifying S. pneumoniae and, if used in conjunction with Gram staining and/or other suitable technique like antigen detection, it may potentially facilitate a fast and accurate diagnosis of pneumococcal infections.
Keywords: Forssman antigen; Streptococcus pneumoniae; binding lectins; fluorescence microscopy; teichoic acids.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

