High-Throughput Chemical Screening Identifies Compounds that Inhibit Different Stages of the Phytophthora agathidicida and Phytophthora cinnamomi Life Cycles
- PMID: 28769905
- PMCID: PMC5515820
- DOI: 10.3389/fmicb.2017.01340
High-Throughput Chemical Screening Identifies Compounds that Inhibit Different Stages of the Phytophthora agathidicida and Phytophthora cinnamomi Life Cycles
Abstract
Oomycetes in the genus Phytophthora are among the most damaging plant pathogens worldwide. Two important species are Phytophthora cinnamomi, which causes root rot in thousands of native and agricultural plants, and Phytophthora agathidicida, which causes kauri dieback disease in New Zealand. As is the case for other Phytophthora species, management options for these two pathogens are limited. Here, we have screened over 100 compounds for their anti-oomycete activity, as a potential first step toward identifying new control strategies. Our screening identified eight compounds that showed activity against both Phytophthora species. These included five antibiotics, two copper compounds and a quaternary ammonium cation. These compounds were tested for their inhibitory action against three stages of the Phytophthora life cycle: mycelial growth, zoospore germination, and zoospore motility. The inhibitory effects of the compounds were broadly similar between the two Phytophthora species, but their effectiveness varied widely among life cycle stages. Mycelial growth was most successfully inhibited by the antibiotics chlortetracycline and paromomycin, and the quaternary ammonium salt benzethonium chloride. Copper chloride and copper sulfate were most effective at inhibiting zoospore germination and motility, whereas the five antibiotics showed relatively poor zoospore inhibition. Benzethonium chloride was identified as a promising antimicrobial, as it is effective across all three life cycle stages. While further testing is required to determine their efficacy and potential phytotoxicity in planta, we have provided new data on those agents that are, and those that are not, effective against P. agathidicida and P. cinnamomi. Additionally, we present here the first published protocol for producing zoospores from P. agathidicida, which will aid in the further study of this emerging pathogen.
Keywords: Agathis australis; avocado root rot; high-throughput screening; kauri dieback; oomycetes; phenotype microarray; zoospore.
Figures
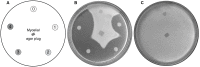
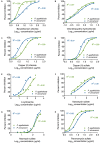

References
-
- Allen R. N., Newhook F. J. (1973). Chemotaxis of zoospores of Phytophthora cinnamomi to ethanol in capillaries of soil pore dimensions. Trans. Br. Mycol. Soc. 61 287–302. 10.1016/S0007-1536(73)80152-4 - DOI
-
- Bauer A. W., Kirby W. M. M., Sherris J. C., Turck M. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 57 592–597. - PubMed
-
- Beever R. E., Walpara N. W., Ramsfield T. D., Dick M. A., Horner I. J. (2009). “Kauri (Agathis australis) under threat from Phytophthora?,” in Phytophthoras in Forests and Natural Ecosystems. Proceedings of the Fourth Meeting of IUFRO Working Party S07.02.09 General Technical report OSW-GTR-221, eds Goheen E. M., Frankel S. J. (Albany, CA: USDA Forest Service; ), 74–85.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

