Plasmid Transfer in the Ocean - A Case Study from the Roseobacter Group
- PMID: 28769910
- PMCID: PMC5513947
- DOI: 10.3389/fmicb.2017.01350
Plasmid Transfer in the Ocean - A Case Study from the Roseobacter Group
Abstract
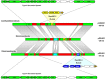
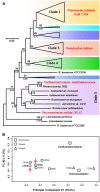
Plasmid mediated horizontal gene transfer (HGT) has been speculated to be one of the prime mechanisms for the adaptation of roseobacters (Rhodobacteraceae) to their ecological niches in the marine habitat. Their plasmids contain ecologically crucial functional modules of up to ∼40-kb in size, e.g., for aerobic anoxygenic photosynthesis, flagellar formation and the biosynthesis of the antibiotic tropodithietic acid. Furthermore, the widely present type four secretion system (T4SS) of roseobacters has been shown to mediate conjugation across genus barriers, albeit in the laboratory. Here we discovered that Confluentimicrobium naphthalenivorans NS6T, a tidal flat bacterium isolated in Korea, carries a 185-kb plasmid, which exhibits a long-range synteny with the conjugative 126-kb plasmid of Dinoroseobacter shibae DFL12T. Both replicons are stably maintained by RepABC operons of the same compatibility group (-2) and they harbor a homologous T4SS. Principal component analysis of the codon usage shows a large similarity between the two plasmids, while the chromosomes are very distinct, showing that neither of the two bacterial species represents the original host of those RepABC-2 type plasmids. The two species do not share a common habitat today and they are phylogenetically only distantly related. Our finding demonstrates the first clear-cut evidence for conjugational plasmid transfer across biogeographical and phylogenetic barriers in Rhodobacteraceae and documents the importance of conjugative HGT in the ocean.
Keywords: conjugation; evolution; horizontal gene transfer; plasmid synteny; type IV secretion systems.
Figures
References
-
- Biebl H., Allgaier M., Tindall B. J., Koblizek M., Lünsdorf H., Pukall R., et al. (2005). Dinoroseobacter shibae gen. nov., sp. nov., a new aerobic phototrophic bacterium isolated from dinoflagellates. Int. J. Syst. Evol. Microbiol. 55 1089–1096. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

