Application of Mycorrhiza and Soil from a Permaculture System Improved Phosphorus Acquisition in Naranjilla
- PMID: 28769964
- PMCID: PMC5515901
- DOI: 10.3389/fpls.2017.01263
Application of Mycorrhiza and Soil from a Permaculture System Improved Phosphorus Acquisition in Naranjilla
Abstract
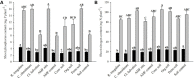
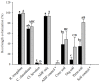
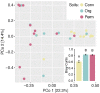
Naranjilla (Solanum quitoense) is a perennial shrub plant mainly cultivated in Ecuador, Colombia, and Central America where it represents an important cash crop. Current cultivation practices not only cause deforestation and large-scale soil degradation but also make plants highly susceptible to pests and diseases. The use of arbuscular mycorrhizal fungi (AMF) can offer a possibility to overcome these problems. AMF can act beneficially in various ways, for example by improving plant nutrition and growth, water relations, soil structure and stability and protection against biotic and abiotic stresses. In this study, the impact of AMF inoculation on growth and nutrition parameters of naranjilla has been assessed. For inoculation three European reference AMF strains (Rhizoglomus irregulare, Claroideoglomus claroideum, and Cetraspora helvetica) and soils originating from three differently managed naranjilla plantations in Ecuador (conventional, organic, and permaculture) have been used. This allowed for a comparison of the performance of exotic AMF strains (reference strains) versus native consortia contained in the three soils used as inocula. To study fungal communities present in the three soils, trap cultures have been established using naranjilla as host plant. The community structures of AMF and other fungi inhabiting the roots of trap cultured naranjilla were assessed using next generation sequencing (NGS) methods. The growth response experiment has shown that two of the three reference AMF strains, a mixture of the three and soil from a permaculture site led to significantly better acquisition of phosphorus (up to 104%) compared to uninoculated controls. These results suggest that the use of AMF strains and local soils as inoculants represent a valid approach to improve nutrient uptake efficiency of naranjilla and consequently to reduce inputs of mineral fertilizers in the cultivation process. Improved phosphorus acquisition after inoculation with permaculture soil might have been caused by a higher abundance of AMF and the presence of Piriformospora indica as revealed by NGS. A higher frequency of AMF and enhanced root colonization rates in the trap cultures supplemented with permaculture soil highlight the importance of diverse agricultural systems for soil quality and crop production.
Keywords: Piriformospora indica; arbuscular mycorrhizal fungi; farming practices; fungal communities; naranjilla; next generation sequencing; permaculture.
Figures

References
-
- Abd-Alla M. H., Omar S. A., Karanxha S. (2000). The impact of pesticides on arbuscular mycorrhizal and nitrogen-fixing symbioses in legumes. Appl. Soil Ecol. 14 191–200. 10.1016/S0929-1393(00)00056-1 - DOI
-
- Achatz B., von Rüden S., Andrade D., Neumann E., Pons-Kühnemann J., Kogel K.-H., et al. (2010). Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil 333 59–70. 10.1007/s11104-010-0319-0 - DOI
-
- Acosta Ó, Pérez A. M., Vaillant F. (2009). Chemical characterization, antioxidant properties, and volatile constituents of naranjilla (Solanum quitoense Lam.) cultivated in Costa Rica. Arch. Latinoam. Nutr. 59 88–94. - PubMed
-
- Antunes P. M., Koch A. M., Dunfield K. E., Hart M. M., Downing A., Rillig M. C., et al. (2009). Influence of commercial inoculation with Glomus intraradices on the structure and functioning of an AM fungal community from an agricultural site. Plant Soil 317 257–266. 10.1007/s11104-008-9806-y - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

