Objective evaluation of chemotherapy-induced peripheral neuropathy using quantitative pain measurement system (Pain Vision®), a pilot study
- PMID: 28770097
- PMCID: PMC5526312
- DOI: 10.1186/s40780-017-0089-4
Objective evaluation of chemotherapy-induced peripheral neuropathy using quantitative pain measurement system (Pain Vision®), a pilot study
Abstract
Background: In an evaluation of chemotherapy-induced peripheral neuropathy (CIPN), objectivity may be poor because the evaluation is determined by the patient's subjective assessment. In such cases, management of neuropathy may be delayed and CIPN symptoms may become severe. In this pilot study, we attempted an objective evaluation of CIPN using a quantitative pain measurement system (Pain Vision®).
Methods: The subjects were patients with gynecologic cancer who underwent chemotherapy using taxane and platinum drugs. The grade of the peripheral sensory nerve disorder was based on the Common Terminology Criteria for Adverse Events (CTC-AE) ver. 4.0 and was evaluated before the initiation of therapy and up to six chemotherapy cycles. A symptom scale assessed by the patients using a peripheral neuropathy questionnaire (PNQ) was also evaluated. Simultaneously during these evaluations, graded electric current was applied from the probe to a fingertip and measured both the lowest perceptible current and lowest current perceived as pain by Pain Vision®. From these values, the pain degree was calculated from the following formula: (pain perception current value - lowest perceptible current value) ÷ lowest perceptible current value × 100. We compared the pain degrees by Pain Vision® during CIPN development with the value obtained before chemotherapy initiation.
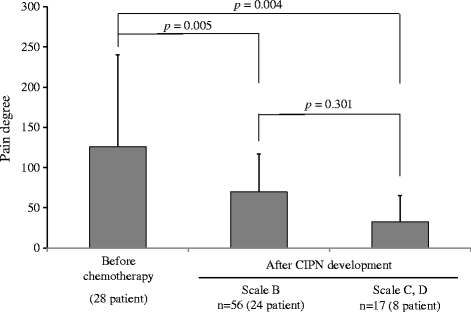
Results: Forty-one patients were enrolled. In the evaluation by a medical professional, 28 (64.3%) patients developed CIPN during 2.5 ± 1.1 chemotherapy cycles (mean ± standard deviation). The pain degree by Pain Vision® at grade 1 and 2 CIPN development according to the evaluation (CTC-AE) was significantly decreased compared to that before chemotherapy initiation (126.0 ± 114.5 vs. 69.8 ± 46.8, p = 0.001, and 126.0 ± 114.5 vs. 32.8 ± 32.6, p = 0.004). Changes in the pain degree by Pain Vision® were also found during scale B and C, D CIPN development in the patient evaluation (PNQ) (115.9 ± 112.4 vs. 70.6 ± 56.5, p = 0.005, and 115.9 ± 112.4 vs. 46.3 ± 42.9, p = 0.004). In the 13 patients in whom CIPN did not occur, no significant decrease in the pain degree by Pain Vision® was detected (p = 0.764). There was no discontinuation of the measurements because of adverse events such as discomfort from the electric current.
Conclusion: The decrease in the pain degree measured by Pain Vision® was associated with the onset of CIPN symptoms. Particularly, detection of CIPN by Pain Vision® was possible, though most of the CIPN that occurred was low grade or mild symptom. Pain Vision® might become a noninvasive and convenient objective CIPN detection tool to supplement subjective CIPN evaluation.
Trial registration: The study approval number in the institution; H25-140. Registered December 17, 2013.
Keywords: Chemotherapy-induced peripheral neuropathy; Objective evaluation; Pain degree; Pain vision; Quantitative pain measurement system.
Conflict of interest statement
Authors’ information
JS. Ph.D.; Master of Hospital Pharmacy, Lecturer of School of Pharmacy, Iwate Medical University, JSPHCS-Certified Oncology Pharmacist (JOP), JSPHCS-Certified Senior Oncology Pharmacist (JSOP), and JPPS-Certified Pharmacist in Palliative Pharmacy (BCPPP). MM; JPPS-Certified Pharmacist in Palliative Pharmacy (BCPPP). SN; JSPHCS-Certified Oncology Pharmacist (JOP). ST. M.D., Ph.D.; Associate Professor of School of Medicine, Iwate Medical University, JSGO-Certified Gynecologic Oncologist, MK M.D., Ph.D.; Assistant director of Breastpia Miyazaki Hospital, JBCS-Certified Medical Specialist. KK. Ph.D.; Proressor, School of Pharmacy, Iwate Medical University.
Ethics approval and consent to participate
This study was conducted with the approval of Iwate Medical University School of Medicine Ethical Review Board (H25–140).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Arakawa K, Torigoe K, Kuzumaki N, Suzuki T, Narita M. Chemothrapy-induced peripheral neuropathy:clinical symptoms, managements and mechanism. Jpn J Pharm Palliat Care Sci. 2011;4:1–13.
-
- Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

