Genomic Signature of the Natural Oncolytic Herpes Simplex Virus HF10 and Its Therapeutic Role in Preclinical and Clinical Trials
- PMID: 28770166
- PMCID: PMC5509757
- DOI: 10.3389/fonc.2017.00149
Genomic Signature of the Natural Oncolytic Herpes Simplex Virus HF10 and Its Therapeutic Role in Preclinical and Clinical Trials
Abstract
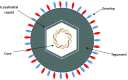
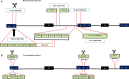
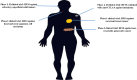
Oncolytic viruses (OVs) are opening new possibilities in cancer therapy with their unique mechanism of selective replication within tumor cells and triggering of antitumor immune responses. HF10 is an oncolytic herpes simplex virus-1 with a unique genomic structure that has non-engineered deletions and insertions accompanied by frame-shift mutations, in contrast to the majority of engineered OVs. At the genetic level, HF10 naturally lacks the expression of UL43, UL49.5, UL55, UL56, and latency-associated transcripts, and overexpresses UL53 and UL54. In preclinical studies, HF10 replicated efficiently within tumor cells with extensive cytolytic effects and induced increased numbers of activated CD4+ and CD8+ T cells and natural killer cells within the tumor, leading to a significant reduction in tumor growth and prolonged survival rates. Investigator-initiated clinical studies of HF10 have been completed in recurrent breast carcinoma, head and neck cancer, and unresectable pancreatic cancer in Japan. Phase I trials were subsequently completed in refractory superficial cancers and melanoma in the United States. HF10 has been demonstrated to have a high safety margin with low frequency of adverse effects in all treated patients. Interestingly, HF10 antigens were detected in pancreatic carcinoma over 300 days after treatment with infiltration of CD4+ and CD8+ T cells, which enhanced the immune response. To date, preliminary results from a Phase II trial have indicated that HF10 in combination with ipilimumab (anti-CTLA-4) is safe and well tolerated, with high antitumor efficacy. Improvement of the effect of ipilimumab was observed in patients with stage IIIb, IIIc, or IV unresectable or metastatic melanoma. This review provides a concise description of the genomic functional organization of HF10 compared with talimogene laherparepvec. Furthermore, this review focuses on HF10 in cancer treatment as monotherapy as well as in combination therapy through a concise description of all preclinical and clinical data. In addition, we will address approaches for future directions in HF10 studies as cancer therapy.
Keywords: HF10; clinical trials; combination therapy; future directions; genomic structure; herpes simplex oncolytic viruses; preclinical studies; talimogene laherparepvec.
Figures

Similar articles
-
[Oncolytic virotherapy using replication-competent herpes simplex viruses].Uirusu. 2007 Jun;57(1):57-65. doi: 10.2222/jsv.57.57. Uirusu. 2007. PMID: 18040155 Review. Japanese.
-
Phase I Dose-escalation Clinical Trial of HF10 Oncolytic Herpes Virus in 17 Japanese Patients with Advanced Cancer.Hepatogastroenterology. 2014 May;61(131):599-605. Hepatogastroenterology. 2014. PMID: 26176043 Clinical Trial.
-
Enhanced cytotoxicity with a novel system combining the paclitaxel-2'-ethylcarbonate prodrug and an HSV amplicon with an attenuated replication-competent virus, HF10 as a helper virus.Cancer Lett. 2010 Feb 1;288(1):17-27. doi: 10.1016/j.canlet.2009.06.014. Epub 2009 Jul 14. Cancer Lett. 2010. PMID: 19604626
-
Determination and analysis of the DNA sequence of highly attenuated herpes simplex virus type 1 mutant HF10, a potential oncolytic virus.Microbes Infect. 2007 Feb;9(2):142-9. doi: 10.1016/j.micinf.2006.10.019. Epub 2006 Dec 12. Microbes Infect. 2007. PMID: 17218138
-
The Current Status and Future Prospects of Oncolytic Viruses in Clinical Trials against Melanoma, Glioma, Pancreatic, and Breast Cancers.Cancers (Basel). 2018 Sep 26;10(10):356. doi: 10.3390/cancers10100356. Cancers (Basel). 2018. PMID: 30261620 Free PMC article. Review.
Cited by
-
Evaluation of parameters for efficient purification and long-term storage of herpes simplex virus-based vectors.Mol Ther Methods Clin Dev. 2022 Jun 13;26:132-143. doi: 10.1016/j.omtm.2022.06.007. eCollection 2022 Sep 8. Mol Ther Methods Clin Dev. 2022. PMID: 35795777 Free PMC article.
-
An Extensive Review on Preclinical and Clinical Trials of Oncolytic Viruses Therapy for Pancreatic Cancer.Front Oncol. 2022 May 24;12:875188. doi: 10.3389/fonc.2022.875188. eCollection 2022. Front Oncol. 2022. PMID: 35686109 Free PMC article. Review.
-
In Situ Cancer Vaccination and Immunovirotherapy Using Oncolytic HSV.Viruses. 2021 Aug 31;13(9):1740. doi: 10.3390/v13091740. Viruses. 2021. PMID: 34578321 Free PMC article. Review.
-
The emerging role of oncolytic virus therapy against cancer.Chin Clin Oncol. 2018 Apr;7(2):16. doi: 10.21037/cco.2018.04.04. Chin Clin Oncol. 2018. PMID: 29764161 Free PMC article. Review.
-
Recent Advances in Molecular Mechanisms of Cancer Immunotherapy.Cancers (Basel). 2023 May 11;15(10):2721. doi: 10.3390/cancers15102721. Cancers (Basel). 2023. PMID: 37345057 Free PMC article. Review.
References
-
- Murphy FA, Fauquet CM, Bishop DH, Ghabrial SA, Jarvis A, Martelli GP, et al. Virus Taxonomy: Classification and Nomenclature of Viruses. New York, NY: Springer-Verlag; (2012).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

